Your How many carbon atoms are in one mole of glucose c6h12o6 images are available in this site. How many carbon atoms are in one mole of glucose c6h12o6 are a topic that is being searched for and liked by netizens today. You can Find and Download the How many carbon atoms are in one mole of glucose c6h12o6 files here. Find and Download all royalty-free vectors.
If you’re looking for how many carbon atoms are in one mole of glucose c6h12o6 images information related to the how many carbon atoms are in one mole of glucose c6h12o6 keyword, you have pay a visit to the ideal site. Our website frequently gives you suggestions for seeing the highest quality video and image content, please kindly search and locate more informative video content and graphics that match your interests.
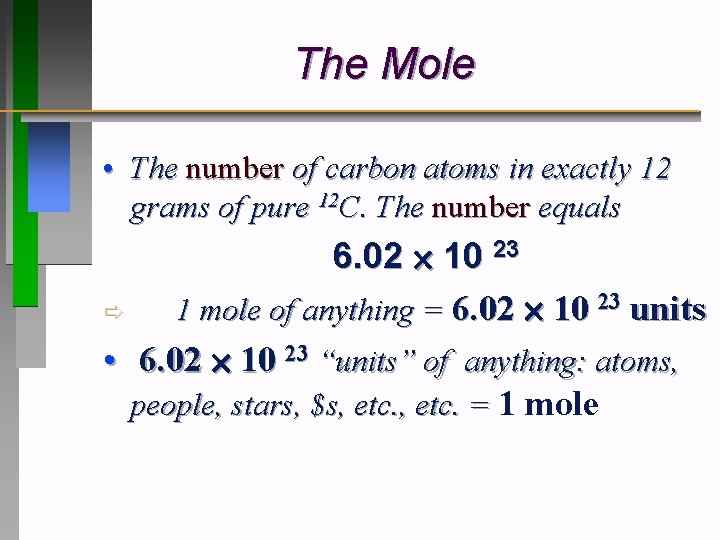
How Many Carbon Atoms Are In One Mole Of Glucose C6h12o6. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. Thats six molecules of Carbon 6 x C C6 plus six molecules of water 6 x H2O H12O6. Can food allergies make eczema worse. There are 6 atoms of C12 atoms of H and 6 atoms of O present in a molecule of glucose C6H12O6.

How many moles of carbon are in C6H12O6. Six carbon atoms twelve hydrogen atoms six oxygen atoms so twenty-four total atoms in one glucose molecule. There are 6 atoms of C12 atoms of H and 6 atoms of O present in a molecule of glucose C6H12O6. What happens to CO2 in the Calvin cycle. One mole of glucose 246022x 10 23 atoms three moles of glucose 3246022x 10 23 atoms Carbon atoms in three moles of glucose 63 6022x 10 23 atoms. From the molecular formula C6H12O6 one can find there are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in one molecule of glucose.
That is going to represent our final answer.
Convert grams C6H12O6 to moles or moles C6H12O6 to grams. There are 6 carbons 12 hydrogens and 6 oxygens so. Six carbon atoms twelve hydrogen atoms six oxygen atoms so twenty-four total atoms in one glucose molecule. The small number after the element symbol is called the s. Accordingly how many molecules of glucose does it contain. What happens to CO2 in the Calvin cycle.

There are 48 atoms in two molecules of glucose 2C6H12O6 12 atoms from. Glucose C6H12O6 is a monosaccharide that contains twelve hydrogenatoms six carbon atoms and six oxygen atoms. From the molecular formula C6H12O6 one can find there are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in one molecule of glucose. The chemical formula for glucose is C6H12O6. The Calvin cycle which takes place in the stroma uses ATP and NADPH to convert carbon dioxide to sugar.

Six carbon atoms twelve hydrogen atoms six oxygen atoms so twenty-four total atoms in one glucose molecule. There are 6 carbons 12 hydrogens and 6 oxygens so. Can food allergies make eczema worse. So we can say that one mole of glucose C6H12O6 contains 602. This compound is also known as Glucose or Fructose or Galactose.

One mole of glucose 6022x 10 23 molecules three moles of glucose 36022x 10 23 molecules In a molecule of glucose there are 24 atoms. This compound is also known as Glucose or Fructose or Galactose. From the molecular formula C6H12O6 one can find there are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in one molecule of glucose. One mole is 6021023 molecules. What happens to CO2 in the Calvin cycle.
 Source: youtube.com
Source: youtube.com
C6H12O6 That means glucose is made of 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms. From the molecular formula C6H12O6 one can find there are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in one molecule of glucose. This compound is also known as Glucose or Fructose or Galactose. How many atoms are in 15 moles Na. Glucose C6H12O6 is a monosaccharide that contains twelve hydrogenatoms six carbon atoms and six oxygen atoms.
 Source: slidetodoc.com
Source: slidetodoc.com
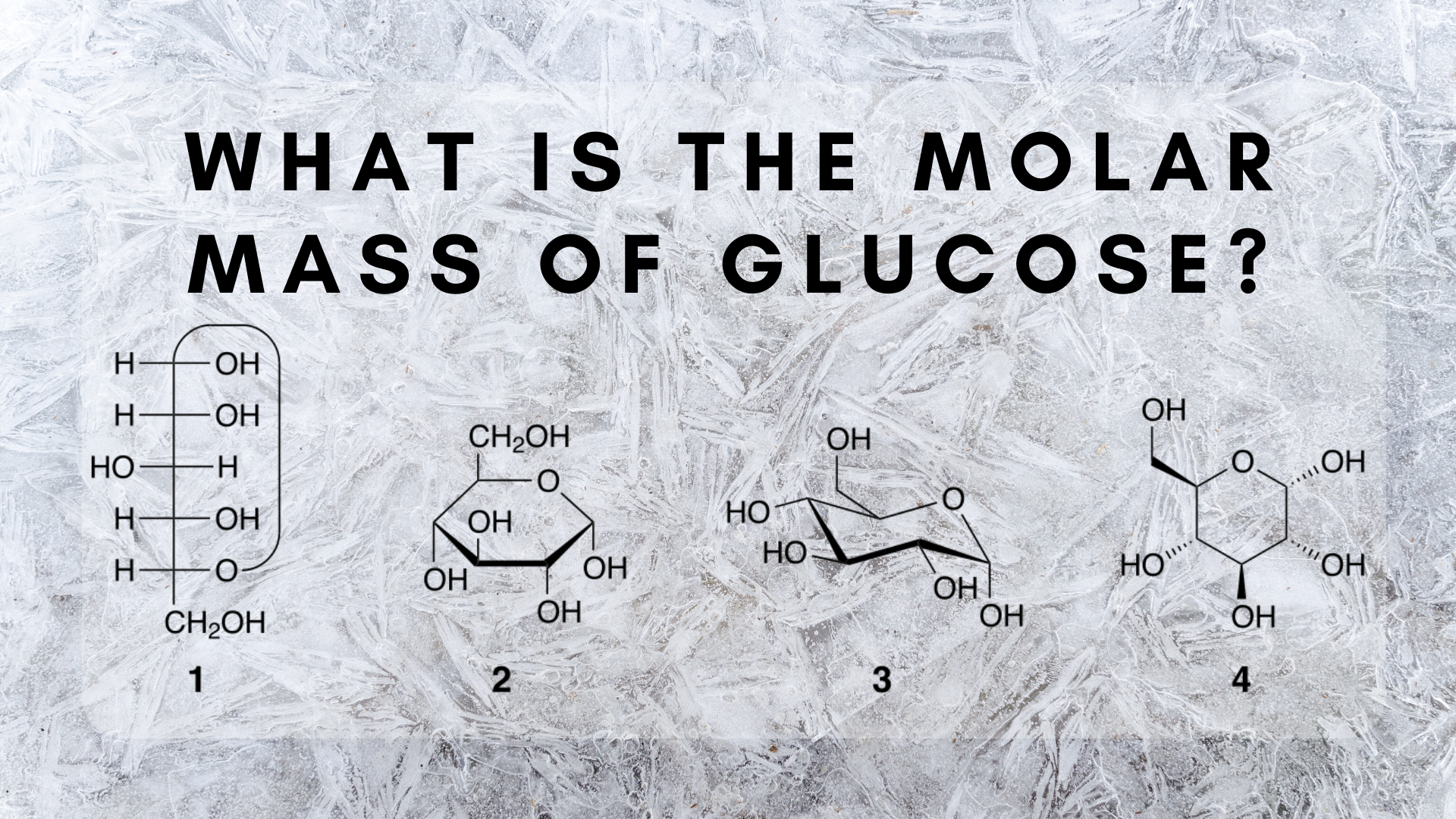
Glucose blood sugar fructose fruit sugar and galactose milk sugar. Molar mass of C6H12O6 18015588 gmol. Six carbon dioxide molecules CO2 are required to create one glucose molecule C6H12O6 because carbon dioxide has one carbon per molecule while glucose molecules have six carbons. How many atoms are there in the molecule C6H12O6. This molecule of the sugar glucose consists of 6 carbon atoms bonded together as a chain with additional atoms of oxygen and hydrogen.

How many atoms are there in C6H12O6. 35 Votes Glucose has a chemical formula of. Thats six molecules of Carbon 6 x C C6 plus six molecules of water 6 x H2O H12O6. You will be building one type of sugar called glucose. There are 6 carbons 12 hydrogens and 6 oxygens so.
 Source: unm.edu
Source: unm.edu
C6H12O6 molecular weight. There are 6 carbons 12 hydrogens and 6 oxygens so. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. Accordingly how many molecules of glucose does it contain. You will be building one type of sugar called glucose.
 Source: unm.edu
Source: unm.edu
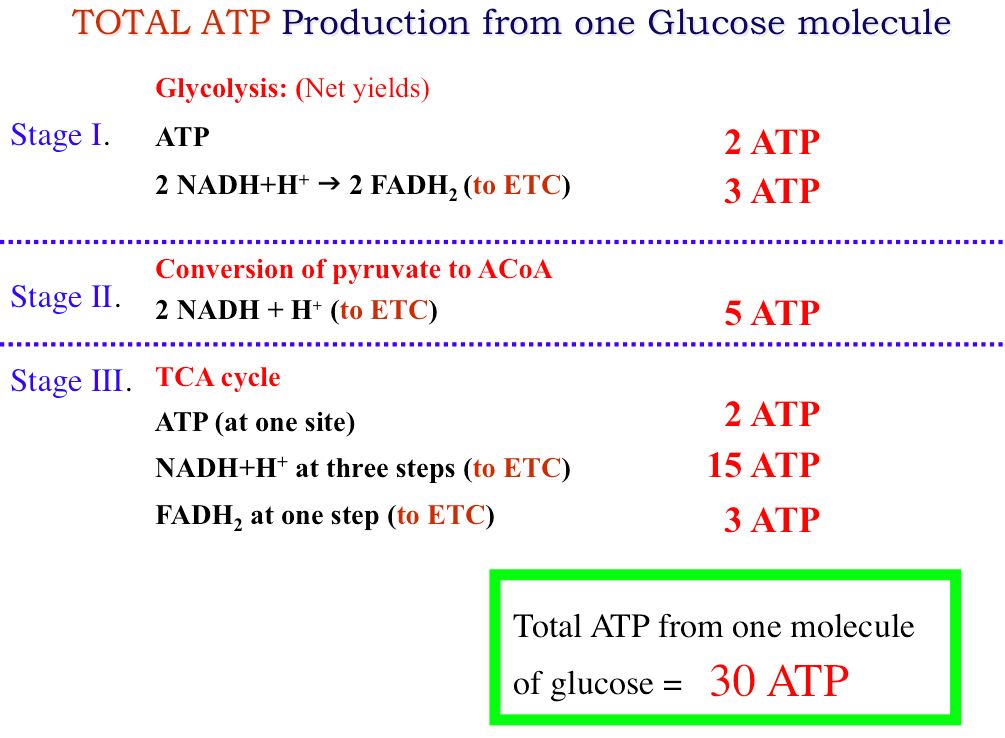
Glucose C6H12O6 is a monosaccharide that contains twelve hydrogenatoms six carbon atoms and six oxygen atoms. Molar mass of C6H12O6 18015588 gmol. Six carbon atoms twelve hydrogen atoms six oxygen atoms so twenty-four total atoms in one glucose molecule. There are 602 x 1023 particles in a mole so 180 g of glucose must contain 6 x 602. Thats six molecules of Carbon 6 x C C6 plus six molecules of water 6 x H2O H12O6.

There are 602 x 1023 particles in a mole so 180 g of glucose must contain 6 x 602. The chemical formula for glucose is C6H12O6. Glucose blood sugar fructose fruit sugar and galactose milk sugar. C6H12O6 That means glucose is made of 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g.
 Source: brainly.in
Source: brainly.in
There are 602 x 1023 particles in a mole so 180 g of glucose must contain 6 x 602. Glucose C6H12O6 is a monosaccharide that contains twelve hydrogenatoms six carbon atoms and six oxygen atoms. What happens to CO2 in the Calvin cycle. The chemical formula for glucose is C6H12O6. How many atoms are in a single.
 Source: numerade.com
Source: numerade.com
How many moles of carbon atoms are in 5 moles of glucose c6h12o6. How many atoms are there in C6H12O6. Six carbon atoms twelve hydrogen atoms six oxygen atoms so twenty-four total atoms in one glucose molecule. This chemical compound has 6 atoms of carbon 12 atoms of hydrogen and 6 atoms of oxygen. C6H12O6 That means glucose is made of.
 Source: youtube.com
Source: youtube.com
This chemical compound has 6 atoms of carbon 12 atoms of hydrogen and 6 atoms of oxygen. Calculate the number of C H and O atoms in 150 g of glucose C6H12O6 a sugar. Watch out a lot more about it. Multiply the moles of glucose present 01 moles in this case by the number of carbon atoms per molecule six to get 06 moles of carbon which translates into about 3611023 carbon atoms. From the molecular formula C6H12O6 one can find there are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in one molecule of glucose.
 Source: youtube.com
Source: youtube.com
One mole of glucose 246022x 10 23 atoms three moles of glucose 3246022x 10 23 atoms Carbon atoms in three moles of glucose 63 6022x 10 23 atoms. The chemical formula for glucose is C6H12O6. Convert grams C6H12O6 to moles or moles C6H12O6 to grams. 1201076 10079412 1599946 Percent composition by element. There are 6 carbons 12 hydrogens and 6 oxygens so.
 Source: sciencetrends.com
Source: sciencetrends.com
In a molecule of glucose C6H12O6 there are 24 atoms 6 from carbon 12 from hydrogen and 6 from oxygen. How many moles of carbon are in C6H12O6. The Calvin cycle which takes place in the stroma uses ATP and NADPH to convert carbon dioxide to sugar. Calculate the number of C H and O atoms in 150 g of glucose C6H12O6 a sugar. C6H12O6 That means glucose is made of.
 Source: wasfa-hd.blogspot.com
Source: wasfa-hd.blogspot.com
The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. So we can say that one mole of glucose C6H12O6 contains 602. The Calvin cycle which takes place in the stroma uses ATP and NADPH to convert carbon dioxide to sugar. The chemical formula for glucose is C6H12O6. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many carbon atoms are in one mole of glucose c6h12o6 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.