Your How many atoms in one mole of nacl images are available. How many atoms in one mole of nacl are a topic that is being searched for and liked by netizens now. You can Download the How many atoms in one mole of nacl files here. Find and Download all royalty-free vectors.
If you’re searching for how many atoms in one mole of nacl pictures information linked to the how many atoms in one mole of nacl keyword, you have pay a visit to the ideal site. Our site frequently gives you hints for seeing the highest quality video and image content, please kindly search and locate more informative video content and images that match your interests.
How Many Atoms In One Mole Of Nacl. 1 grams NaCl is equal to 0017110756386119 mole. What is the mole equation. You can view more details on each measurement unit. Explanationbut in ONE mole quantity of sodium carbonate there are TWO moles of sodium atoms with a mass of 46g one mole of carbon atoms with a mass of 12g and THREE moles of oxygen atoms with a mass of of 48g.
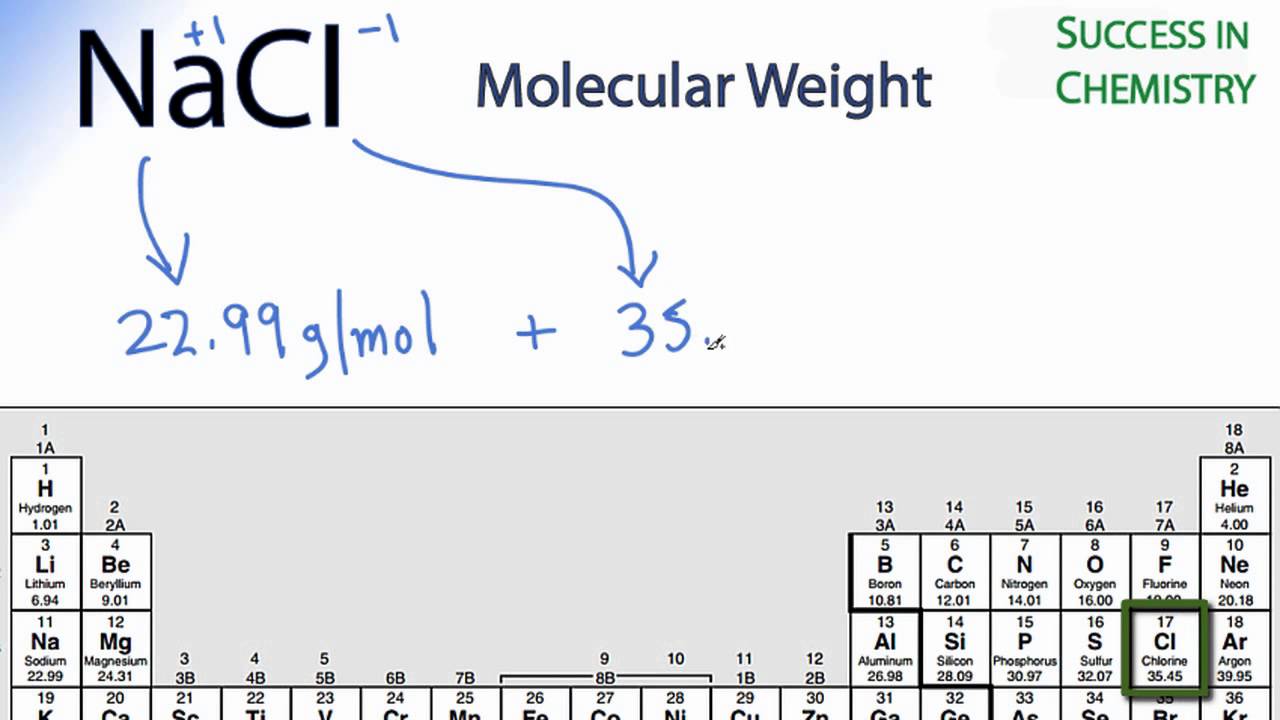
 Mole Calculations Moles To Mass Find The Mass Of One Mole Of Nacl 58 5g Find The Mass Of Two Moles Of Nacl 117 0g How Did You Get The Answer 2 Moles From slideplayer.com
Mole Calculations Moles To Mass Find The Mass Of One Mole Of Nacl 58 5g Find The Mass Of Two Moles Of Nacl 117 0g How Did You Get The Answer 2 Moles From slideplayer.com
This is Avogadros number. 60231023 atoms of Cl 1 mol of Cl. Thus 1 mole of NaCl will contain 6023 x 1023 atoms each of Na and Cl or a total of 12046 x 1023 atoms. You can view more details on each measurement unit. This compound is also known as Sodium Chloride. Avogadros number is a very important relationship to remember.
Sodium Chloride or NaCl is made up of two elements sodium or Na and chlorine or Cl.
Hence each molecule of NaCl has 2 atoms total. Just so how many atoms are in a mole of sodium. Use the mole quantity to count formulas by weighing them. 1 grams NaCl is equal to 0017110756386119 mole. To find moles divide atoms by Avogadros number 511x10 23 atoms of lithium is equal to how many moles. What is the mole equation.
 Source: pinterest.com
Source: pinterest.com
If you have 50 x 1028 atoms of aluminum how many moles is this. The answer is 5844277. Mass of a mole of particles mass of 1 particle x 6022 x 1023 The mass of an atom in amu is numerically the same as the mass of one mole of atoms of the element in grams. Note that rounding errors may occur so always check the results. Thus 1 mole of NaCl will contain 6023 x 1023 atoms each of Na and Cl or a total of 12046 x 1023 atoms.
 Source: howtodiscuss.com
Source: howtodiscuss.com
The mole abbreviated mol is an SI unit which measures the number of particles in a specific substance. One atom of sulfur has a mass of 3207 amu. There are ______ number of molecules atoms of NaCl in one mole of NaCl. Use the mole quantity to count formulas by weighing them. 60231023 atoms of Na 1 mol of Na.
 Source: youtube.com
Source: youtube.com
The meaning and usefulness of the mole Page 2 2 One mole of NaCl contains 6022 x 1023 NaCl formula units. What is the mole equation. 44 so the charge balances. To find moles divide atoms by Avogadros number 511x10 23 atoms of lithium is equal to how many moles. 1 mole 60221023 6022 10 23 atoms molecules protons etc.
 Source: youtube.com
Source: youtube.com
In 1 mole of NaCl there are 602 x 1023 formula units. The 6 face-centered Cl atoms are shared with 2 cubes. One mole is equal to 6022141791023 atoms or other elementary units such as molecules. This is the same as the number of sodium ions in 1 mol of sodium chloride. 60231023 molecules of NaCl 1 mol of NaCl.
 Source: pinterest.com
Source: pinterest.com
A mole is Avagadros number. Sodium Chloride or NaCl is made up of two elements sodium or Na and chlorine or Cl. Since 1 molecule contains 1 atom each of Na and Cl 1 mole of NaCl will contain 1 mole each of Na and Cl. Type in your own numbers in the form to convert the units. To calculate the number of atoms in two moles of sodium use dimensional analysis.
 Source: pinterest.com
Source: pinterest.com
A molecule of sodium chloride NaCl consists of one atom each of sodium and chlorine. 1 grams NaCl is equal to 0017110756386119 mole. This compound is also known as Sodium Chloride. 1 mole is equal to 1 moles NaCl or 5844277 grams. The mass of an atom in amu is numerically the same as the mass of one mole of atoms of the element in grams.
 Source: in.pinterest.com
Source: in.pinterest.com
How many formula units are in 15 moles of NaCl. One mol of NaCl 602 x1023 formulas has a mass of 5844 g. 1 mole is equal to 1 moles NaOH or 3999711 grams. If 1 mole of atoms is 602 x 1023 atoms then 2 moles of atoms would be equal to 12. To calculate the number of atoms in two moles of sodium use dimensional analysis.
 Source: pinterest.com
Source: pinterest.com
Mass of a mole of particles mass of 1 particle x 6022 x 1023 The mass of an atom in amu is numerically the same as the mass of one mole of atoms of the element in grams. 1 mole is equal to 1 moles NaOH or 3999711 grams. The 6 face-centered Cl atoms are shared with 2 cubes. We assume you are converting between grams NaCl and mole. Note that rounding errors may occur so always check the results.
 Source: pinterest.com
Source: pinterest.com
If you have 50 x 1028 atoms of aluminum how many moles is this. One mole of a substance is defined by Avogadro as consisting of 6022 x 1023 atoms. This compound is also known as Sodium Chloride. 60231023 molecules of NaCl 1 mol of NaCl. 1 mole 60221023 6022 10 23 atoms molecules protons etc.
 Source: youtube.com
Source: youtube.com
How many moles are in 602 x 1024 atoms of carbon. 44 so the charge balances. How many moles are in 35 x 1024 atoms of neon. 3 mol Na 60221023 atoms Na1mol Na 18071024 atoms Na. In 1 mole of carbon there are 602 x 1023 carbon atoms.

We know that 1 mole of anything contains an Avogadros number of atoms or 6023 x. The mass of an atom in amu is numerically the same as the mass of one mole of atoms of the element in grams. 60231023 molecules of NaCl 1 mol of NaCl. The mole abbreviated mol is an SI unit which measures the number of particles in a specific substance. 20 moles Na x 60221023g1mol1201024 atoms of Na.
 Source: pinterest.com
Source: pinterest.com
Because technically NaCl is made up of 2 atoms permolecule. Thus 1 mole of NaCl will contain 6023 x 1023 atoms each of Na and Cl or a total of 12046 x 1023 atoms. How many moles are in 301 x 1023 atoms of lithium. We assume you are converting between grams NaCl and mole. Beside above how many atoms are there in 334 moles of sodium.
 Source: slideplayer.com
Source: slideplayer.com
Use the mole quantity to count formulas by weighing them. Each of the 12 edge Na atoms is shared with 4 cubes 3 not shown. How many formula units are in 15 moles of NaCl. 60231023 atoms of Cl 1 mol of Cl. This is Avogadros number.
 Source: pinterest.com
Source: pinterest.com
20 moles Na x 60221023g1mol1201024 atoms of Na. A molecule of sodium chloride NaCl consists of one atom each of sodium and chlorine. How many moles are in 301 x 1023 atoms of lithium. This is Avogadros number. To find moles divide atoms by Avogadros number 511x10 23 atoms of lithium is equal to how many moles.
 Source: chemiris.labs.brocku.ca
Source: chemiris.labs.brocku.ca
The SI base unit for amount of substance is the mole. Note that rounding errors may occur so always check the results. How many moles are in 602 x 1024 atoms of carbon. How many moles are in 301 x 1023 atoms of lithium. Molecule of H2O 180 amu Avagadros number of molecules 602 x1023 1 mol H2O 180 g laboratory sample size H O H Measuring Matter with Moles Slide 33 158.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many atoms in one mole of nacl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






