Your How many atoms in 1 mole of carbon images are ready in this website. How many atoms in 1 mole of carbon are a topic that is being searched for and liked by netizens now. You can Get the How many atoms in 1 mole of carbon files here. Get all royalty-free vectors.
If you’re looking for how many atoms in 1 mole of carbon pictures information linked to the how many atoms in 1 mole of carbon interest, you have pay a visit to the right blog. Our site always gives you hints for downloading the highest quality video and image content, please kindly hunt and locate more informative video articles and images that fit your interests.
How Many Atoms In 1 Mole Of Carbon. How many atoms does 4 CO2 have. 1 grams Carbon is equal to 0083259093974539 mole. Hence we can conclude that there are atoms of each carbon and oxygen atom present in 15 mole of. Note that rounding errors may occur so always check the results.
 Mole Concept Mole Concept Concept Mole From br.pinterest.com
Mole Concept Mole Concept Concept Mole From br.pinterest.com
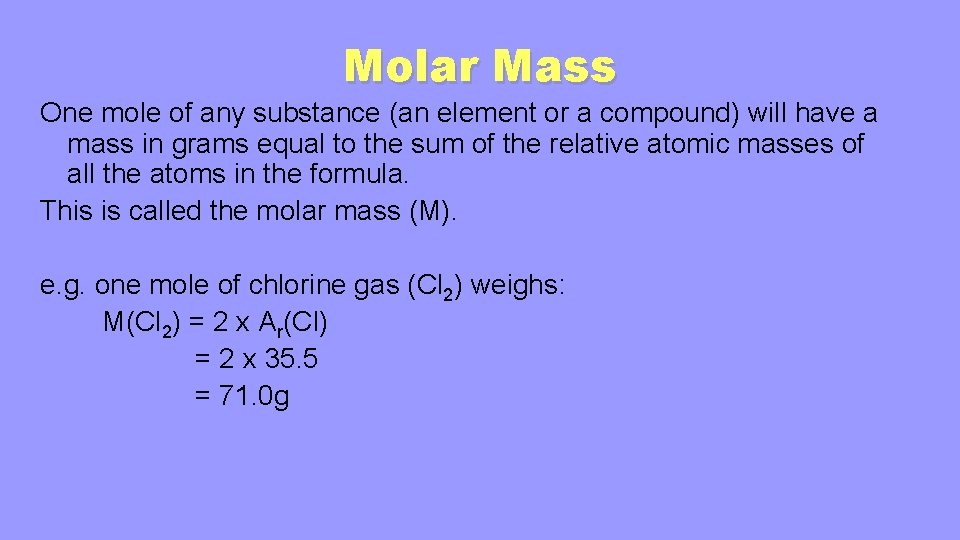
1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. So 1 mole of sucrose contains 12 moles of carbon atoms 22 moles of hydrogen atoms and 11 moles of oxygen atoms. 1 mole of carbon. And number of atoms number of moles times N A 01 N A atoms N A 6022 10 23 01 6022 10 23. 1 mole carbon dioxide contains 602 x 1023 molecules. You can view more details on each measurement unit.
Hence the number of atoms in 01 m o l of carbon atom is 01 N A atoms or 6022 10 22 atoms.
1511024 molecule of CO2 will contain 4521024 atoms. 1 mole 602 1023 particles such as atoms ions or molecules Name ________________________________________________________________ Period Mole. Get the detailed answer. Exactly 12 grams of pure carbon-12 powder is known as one mole. The molecular formula for Carbon is C. In CARBON material it has also 60221023 atoms in one mole of it that is equivalent to 12 grams as it has atomic weight 12amu.
 Source: pinterest.com
Source: pinterest.com
1 mole carbon dioxide contains 602 x 1023 molecules. Also Know how many atoms are there in one mole of carbon. The number of atoms of carbon-12 present in this one mole sample is 6022 136 7 x 1023. 1 mole of carbon. So i choose B.
 Source: pinterest.com
Source: pinterest.com
The subscripts following each element symbol indicate the number of atoms of each element in the molecule. Therefore 250 mole of CO2 will contain 4521024 atoms. Use this page to learn how to convert between moles and atoms. Get the detailed answer. The number of atoms of carbon-12 present in this one mole sample is 6022 136 7 x 10 23This number is known as Avogadros number.
 Source: pinterest.com
Source: pinterest.com
How many atoms of carbon are in 52 mol of carbon. We assume you are converting between grams Carbon and mole. We know it has the formula CO2 and this tells us that. 1 mole of a substance 6022 x 1023 moleculesSo 1 mole 3 atomsmolecule 6022 x 1023 moleculesmole 18066. This number is known as Avogadros number.
 Source: pinterest.com
Source: pinterest.com
1 mole of a substance 6022 x 1023 moleculesSo 1 mole 3 atomsmolecule 6022 x 1023 moleculesmole 18066. There are 2 carbon atoms in the formula. Related Links How Many Different Words Can Be Formed By Jumbling The Letters In The Word Mississippi. 1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. You can view more details on each measurement unit.
 Source: pinterest.com
Source: pinterest.com
To obtain the number of atoms just use the the formula nNLA. 6022 10 22 atoms. So calculate number of atoms in 15 mole as follows. Since there are 2 carbon atoms per C2H6 we must multiply the number of moles of C2H6 by 2 to get the number of moles of Carbon which is 144 or 14 if using two sig figs. Also Know how many atoms are there in one mole of carbon.
 Source: pinterest.com
Source: pinterest.com
6022 10 22 atoms. The subscripts following each element symbol indicate the number of atoms of each element in the molecule. There are three atoms in the compound CO2 one carbon and two oxygen atoms. Hence we can conclude that there are atoms of each carbon and oxygen atom present in 15 mole of. Each elements contains 602210 23 atoms in one mole of their subsatnces.
 Source: pinterest.com
Source: pinterest.com
When dealing with moles and atoms you always use that formula. So 1 mole of sucrose contains 12 moles of carbon atoms 22 moles of hydrogen atoms and 11 moles of oxygen atoms. Type in your own numbers in the form to convert the units. The molecular formula for Carbon is C. Therefore 250 mole of CO2 will contain 4521024 atoms.
 Source: in.pinterest.com
Source: in.pinterest.com
Exactly 12 grams of pure carbon-12 powder is known as one mole. O2z1qpv and 83 more users found this answer helpful. We know it has the formula CO2 and this tells us that. 1 mole of carbon. Since there are 2 carbon atoms per C2H6 we must multiply the number of moles of C2H6 by 2 to get the number of moles of Carbon which is 144 or 14 if using two sig figs.
 Source: pinterest.com
Source: pinterest.com
1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. Related Links How Many Different Words Can Be Formed By Jumbling The Letters In The Word Mississippi. Also Know how many atoms are there in one mole of carbon. 1511024 molecule of CO2 will contain 4521024 atoms. Hence we can conclude that there are atoms of each carbon and oxygen atom present in 15 mole of.
 Source: pinterest.com
Source: pinterest.com
Therefore the answer is 3 atoms x 4 moles x 602214179 x 1023 atomsmole 7226570148 x 1023 atoms. The number of atoms of carbon-12 present in this one mole sample is 6022 136 7 x 1023. The molecular formula for Carbon is C. Hence the number of atoms in 01 m o l of carbon atom is 01 N A atoms or 6022 10 22 atoms. 1 molecule of CO2 contains 3 atoms so.
 Source: pinterest.com
Source: pinterest.com
So in total there are three atoms. We assume you are converting between grams Carbon and mole. Exactly 12 grams of pure carbon-12 powder is known as one mole. Therefore there are 6022 x 1023 atoms of carbon and 12044 x. Molecular weight of Carbon or mol.
 Source: pinterest.com
Source: pinterest.com
1 mole of a substance 6022 x 1023 moleculesSo 1 mole 3 atomsmolecule 6022 x 1023 moleculesmole 18066. Also Know how many atoms are there in one mole of carbon. One molecule of CO2 contains one atom of carbon and two atoms of oxygen One mole of CO2 contains one mole of carbon atoms and two moles of oxygen atoms. So i choose B. The value of the mole in precisely 12 grammes of pure carbon-12 is equal to the number of atoms.
 Source: br.pinterest.com
Source: br.pinterest.com
Exactly 12 grams of pure carbon-12 powder is known as one mole. So 1 mole of sucrose contains 12 moles of carbon atoms 22 moles of hydrogen atoms and 11 moles of oxygen atoms. Each elements contains 60221023 atoms in one mole of their subsatnces. There are three atoms in the compound CO2 one carbon and two oxygen atoms. The subscripts following each element symbol indicate the number of atoms of each element in the molecule.
 Source: br.pinterest.com
Source: br.pinterest.com
Each elements contains 602210 23 atoms in one mole of their subsatnces. 1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. Related Links How Many Different Words Can Be Formed By Jumbling The Letters In The Word Mississippi. The value of the mole in precisely 12 grammes of pure carbon-12 is equal to the number of atoms. We know it has the formula CO2 and this tells us that.
 Source: pinterest.com
Source: pinterest.com
Grams divided by the molar mass of C2H6 which is 180584gmol 722 moles of C2H6. Therefore there are 6022 x 1023 atoms of carbon and 12044 x. Get the detailed answer. 1 mole of carbon. And number of atoms number of moles times N A 01 N A atoms N A 6022 10 23 01 6022 10 23.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many atoms in 1 mole of carbon by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.