Your How many atoms are present in 1 mole of copper images are ready in this website. How many atoms are present in 1 mole of copper are a topic that is being searched for and liked by netizens today. You can Find and Download the How many atoms are present in 1 mole of copper files here. Download all royalty-free photos.
If you’re looking for how many atoms are present in 1 mole of copper images information related to the how many atoms are present in 1 mole of copper interest, you have pay a visit to the right site. Our site always provides you with hints for downloading the maximum quality video and picture content, please kindly search and locate more enlightening video content and images that match your interests.
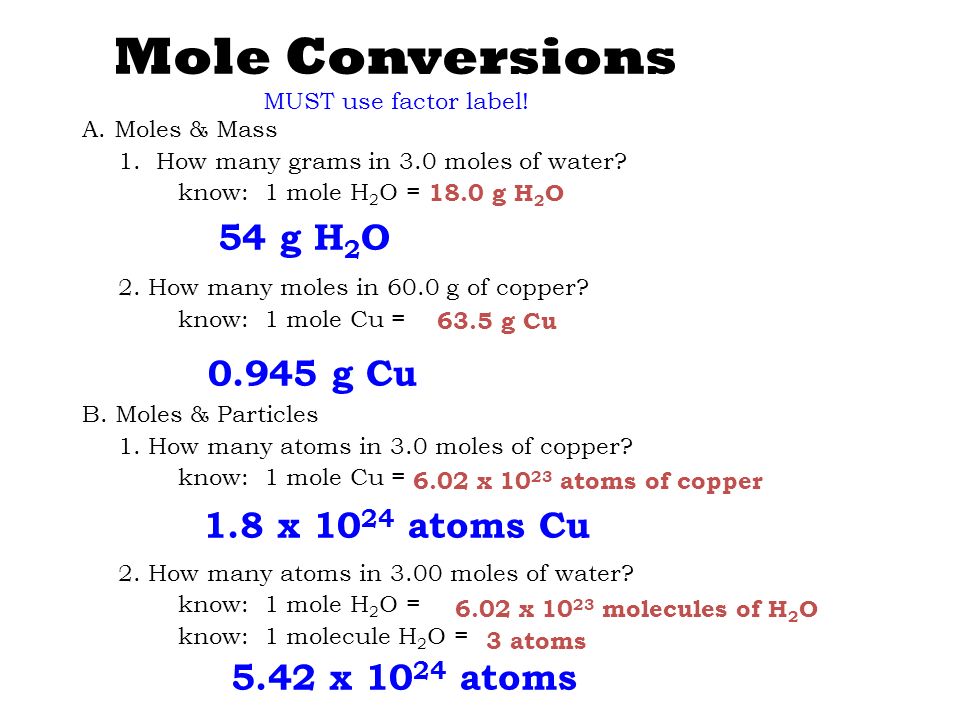
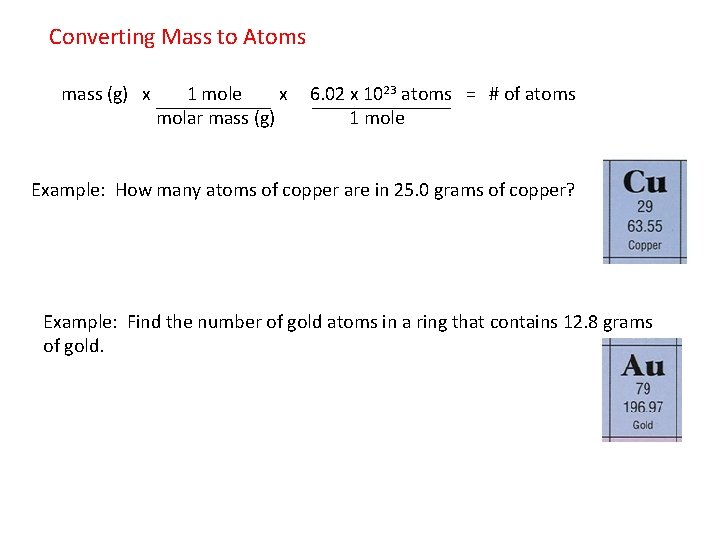
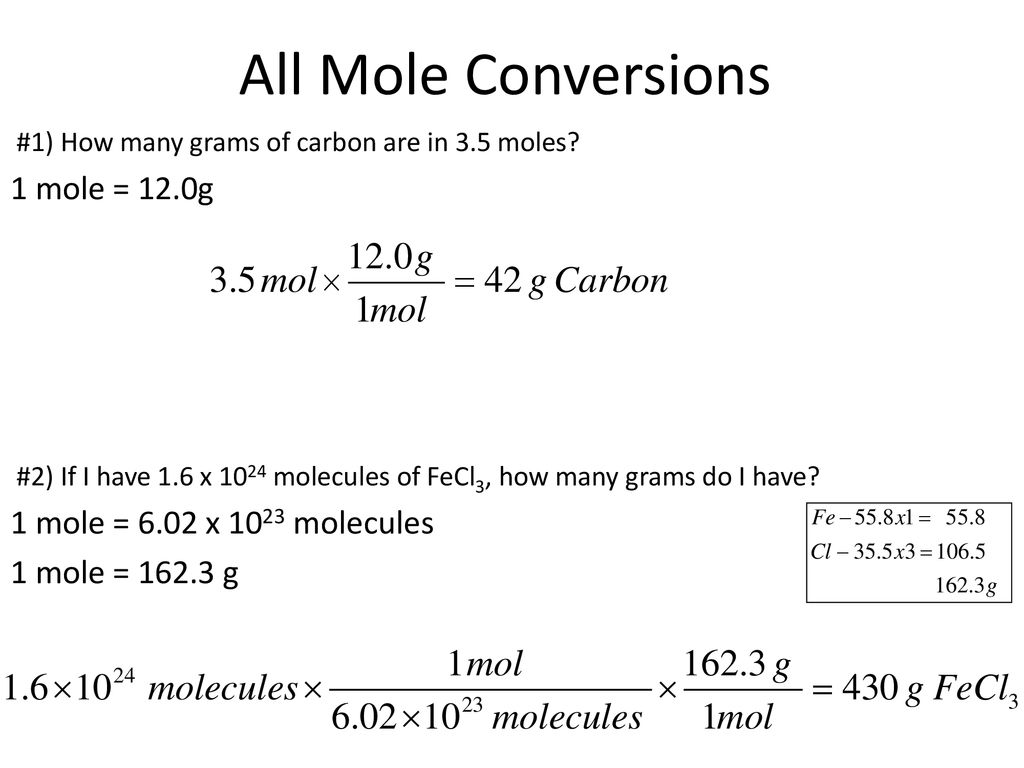
How Many Atoms Are Present In 1 Mole Of Copper. 1 mole is equal to 1 moles Copper or 63546 grams. 425 x 1026 atoms of barium 1 mol 706 mol 602 x 1023 atoms 3. 10 g gold 1 mole 602 x 1023 atoms 306 x 1022 atoms 1970g 1 mole PRACTICE PROBLEMS. Considering that the provided sample mass 500 g is a little less than one-tenth the mass of 1 mole of Cu 64 g a reasonable estimate for the number of atoms in the sample would be on the order of one-tenth N A or approximately 10 22 Cu atoms.
 Chemical Quantities The Mole Ppt Download From slideplayer.com
Chemical Quantities The Mole Ppt Download From slideplayer.com
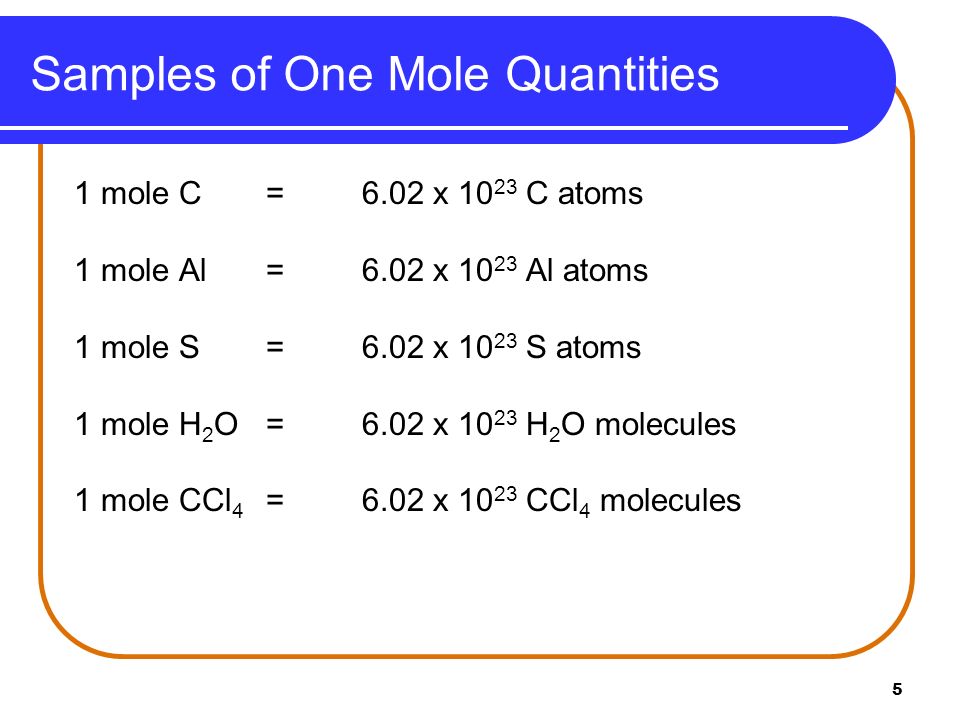
As a result 1 mole of copper contains 6022 1023 atoms of copper. We assume you are converting between moles Copper and gram. How many moles of barium are in a sample containing 425 x 1026 atoms of barium. Molecules H 2 O 4499 g H. 0200 mole of H 2 O contains how many molecules. 0094 moles of Cu.
10 g gold 1 mole 602 x 1023 atoms 306 x 1022 atoms 1970g 1 mole PRACTICE PROBLEMS.
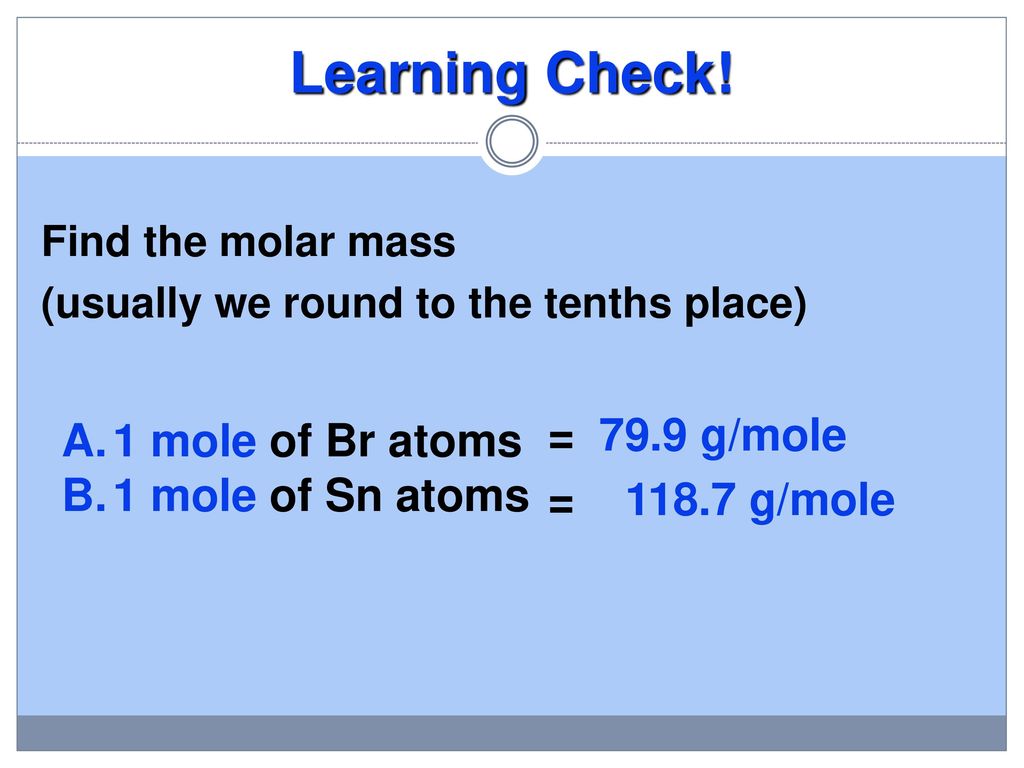
The SI base unit for amount of substance is the mole. Exactly 12 g of carbon-12 contains 6022 x 10 23 atoms. How many atoms of oxygen are in 82 x 1023 formula units of cupric sulfate. 1 mole is equal to 1 moles Copper or 63546 grams. 1 Mole 60221415E23 Atom. The amount of a substance that contains the same number of entities as there are atoms in 12 g of carbon-12.
 Source: pinterest.com
Source: pinterest.com
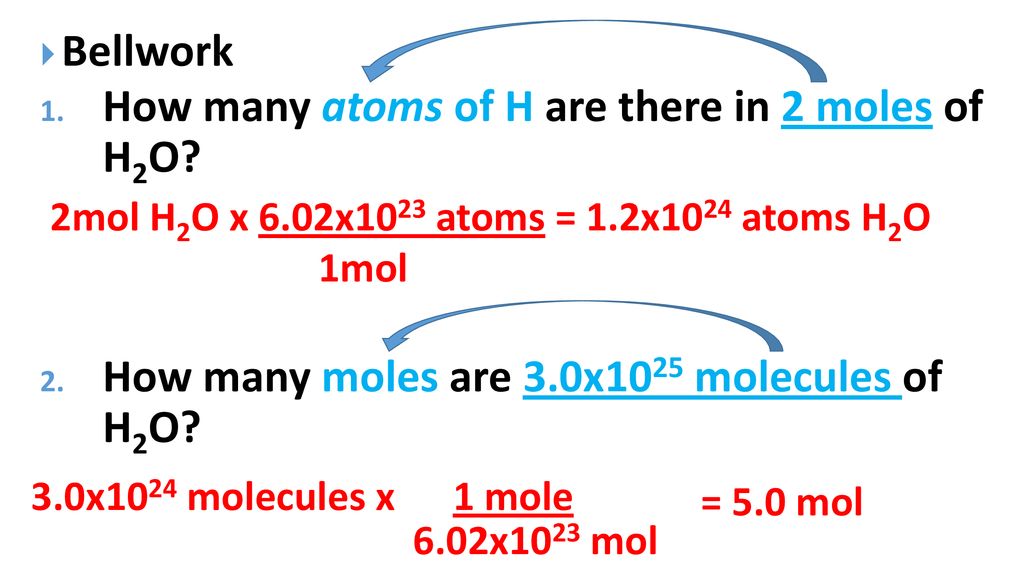
6021023 atoms Cu 1 mol Cu 1751023 atoms Cu How many formula units are in a sample of salt with a mass of 6769 grams. How many moles is 78 x 1025 molecules of phosphorus pentachloride. The unit on Avogadros Number might look a bit weird. Quantity used by chemists with a value of 6022 x 1023 2. The answer is 0015736631731344.
 Source: slidetodoc.com
Source: slidetodoc.com
Molecules H 2 O 4499 g H. From your Periodic Table we learn that one mole of copper 60221023 individual copper atoms have a mass of 6355g. The amount of a substance that contains the same number of entities as there are atoms in 12 g of carbon-12. The answers including units to Examples 1 and 2. Number of moles in 6 grams 6g x 1 mole 63546g.
 Source: slideplayer.com
Source: slideplayer.com
0094 moles of Cu. 602 10 23 copper atoms. The amount of a substance that contains the same number of entities as there are atoms in 12 g of carbon-12. 1 mole of copper. 1 mole of Cu atoms contains 6022 x 1023 atoms of Cu.
 Source: slideplayer.com
Source: slideplayer.com
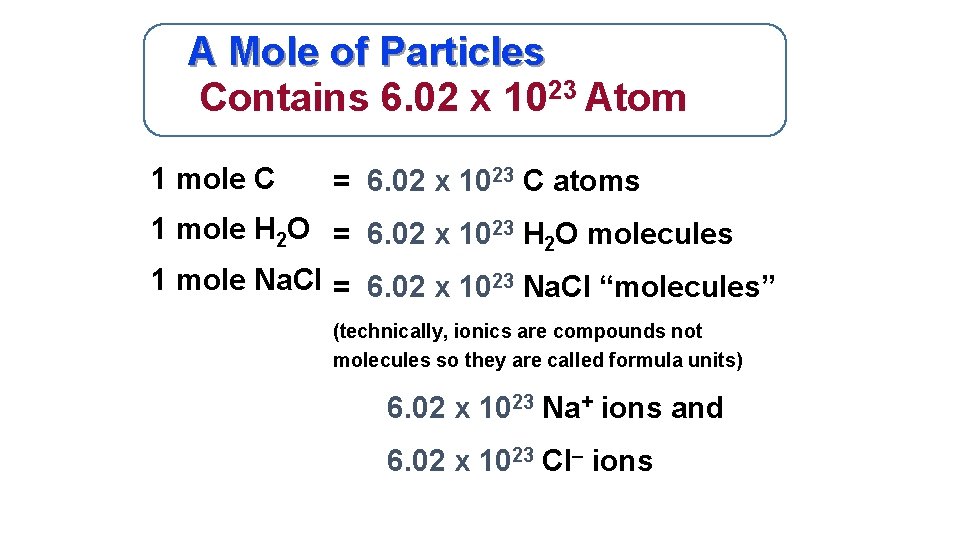
Start at the same box as Example 1. 1 Mole 60221415E23 Atom. Hence it can be easily be deduced that 1 gram atom of sodium will be 23 grams. 0200 mol x 6022 x 10 23 mol 1 see below for answer. 1 mole of sodium is well known to represent a mass of 1 mole of sodium atoms which is 23 grams.
 Source: slideplayer.com
Source: slideplayer.com
3 on a question. Patulomg po pls sige na How many moles of copper is 36310²³ atoms of copperHow many atoms of carbon are present in 254 moles of carbon gas moleculeHow many chlorine ion is present in a moleHow many atoms of carbon are present in carbon gas moleculeHow many moles of carbon atoms and chlorine atoms do it take to make. The number of particles per mole 602 10 23 mol -1 is determined experimentally and is known as the Avogadro constant or the Avogadro number. The molecular formula for Copper is Cu. How many moles are equal to 625g of copper.
 Source: slideplayer.com
Source: slideplayer.com
Exactly 12 g of carbon-12 contains 6022 x 10 23 atoms. 1 grams Copper is equal to 0015736631731344 mole. There fore since the number of atoms in 1 mole is 6022 x 1023 atoms per mole. How many moles of barium are in a sample containing 425 x 1026 atoms of barium. 1 mole of Cu atoms contains 6022 x 1023 atoms of Cu.
 Source: slidetodoc.com
Source: slidetodoc.com
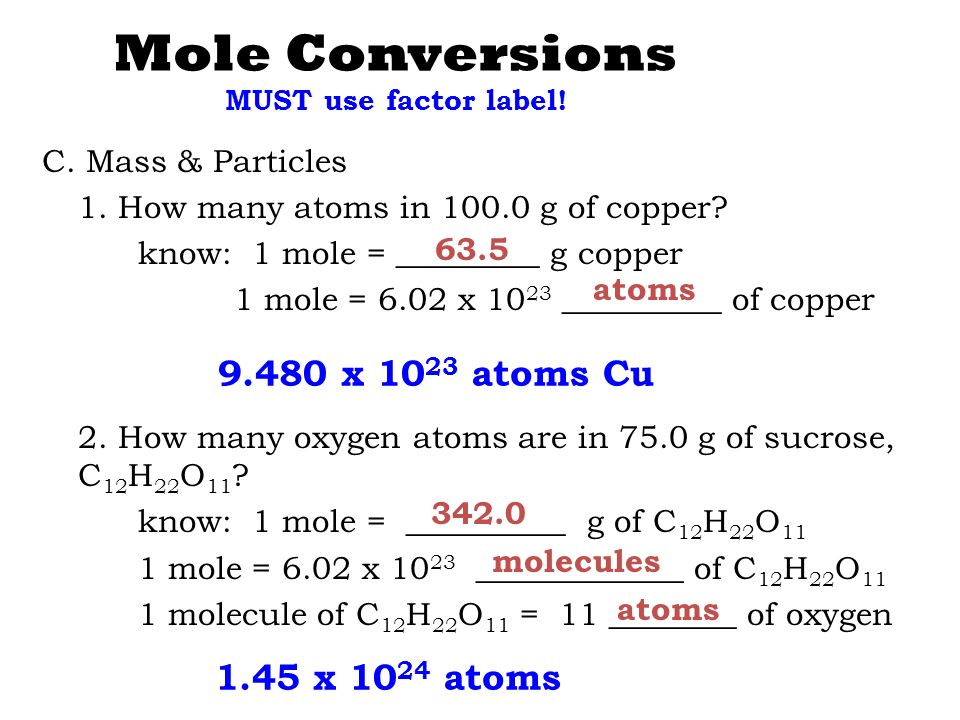
3 rows Since 1 mole of copper weights 6355 gram. Where Atom Number of atoms. 681 grams copper 1 mole Cu6355 grams6022 X 10231 mole Cu 645 X 1022 atoms of copper —–. 1 The mole or mol represents a certain number of objects. 1 mole of Cu atoms contains 6022 x 1023 atoms of Cu.
 Source: slideplayer.com
Source: slideplayer.com
1 mole of sodium is well known to represent a mass of 1 mole of sodium atoms which is 23 grams. One mol of NaCl 602 x1023 formulas has a mass of 5844 g. 1 mole of Cu atoms contains 6022 x 1023 atoms of Cu. There fore since the number of atoms in 1 mole is 6022 x 1023 atoms per mole. We assume you are converting between grams Copper and mole.
 Source: slideplayer.com
Source: slideplayer.com
1 mole of sodium is well known to represent a mass of 1 mole of sodium atoms which is 23 grams. 602 10 23 copper atoms. 1 mole is equal to 1 moles Copper or 63546 grams. One mole of CO2 gas contains 1 mole of carbon atoms and 2 moles of oxygen atoms. The molar mass of Cu 63546 grams per mole.
 Source: slidetodoc.com
Source: slidetodoc.com
The SI base unit for amount of substance is the mole. The answer is 0015736631731344. This means 1 mole of CU atoms has a mass of 63546 grams. The number of particles per mole 602 10 23 mol -1 is determined experimentally and is known as the Avogadro constant or the Avogadro number. We assume you are converting between moles Copper and gram.
 Source: slideplayer.com
Source: slideplayer.com
Mass of one mole of atoms of an element or one mole of molecules or formula units of a. 681 grams copper 1 mole Cu6355 grams6022 X 10231 mole Cu 645 X 1022 atoms of copper —–. 6021023 atoms Cu 1 mol Cu 1751023 atoms Cu How many formula units are in a sample of salt with a mass of 6769 grams. It is mol 1 and you would say per mole out loud. 1 mole of copper.
 Source: chem.libretexts.org
Source: chem.libretexts.org
NaCl 6769 g NaCl. How many atoms of oxygen are present in 500 moles of. One mol of NaCl 602 x1023 formulas has a mass of 5844 g. 1 Mole 60221415E23 Atom. NaCl 6769 g NaCl.
 Source: studylib.net
Source: studylib.net
2 moles of copper. How many atoms of oxygen are present in 500 moles of. 1 mole contains 6022 x 10. The number of particles per mole 602 10 23 mol -1 is determined experimentally and is known as the Avogadro constant or the Avogadro number. 10 g gold 1 mole 602 x 1023 atoms 306 x 1022 atoms 1970g 1 mole PRACTICE PROBLEMS.
 Source: slideplayer.com
Source: slideplayer.com
Molecular weight of Copper or grams. 1 mole of Cu atoms contains 6022 x 1023 atoms of Cu. Start at the same box as Example 1. The unit on Avogadros Number might look a bit weird. How much is a mole of copper.
 Source: slideplayer.com
Source: slideplayer.com
How many atoms of oxygen are in 82 x 1023 formula units of cupric sulfate. Considering that the provided sample mass 500 g is a little less than one-tenth the mass of 1 mole of Cu 64 g a reasonable estimate for the number of atoms in the sample would be on the order of one-tenth N A or approximately 10 22 Cu atoms. 1 Mole 60221415E23 Atom. Mass of a compound 4. 0200 mole of H 2 O contains how many molecules.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many atoms are present in 1 mole of copper by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






