Your How many atoms are in one mole of silver images are ready in this website. How many atoms are in one mole of silver are a topic that is being searched for and liked by netizens now. You can Download the How many atoms are in one mole of silver files here. Find and Download all free vectors.
If you’re looking for how many atoms are in one mole of silver images information related to the how many atoms are in one mole of silver topic, you have come to the right blog. Our site always gives you hints for downloading the highest quality video and picture content, please kindly search and find more informative video content and graphics that match your interests.
How Many Atoms Are In One Mole Of Silver. A 623 10 24 silver atoms B 226 10 24 silver atoms C 161 10 23 silver atoms D 244 10 26 silver atoms E 650 10 25 silver atoms Answer. Molecular weight of C2H5OH or mol The SI base unit for amount of substance is the mole. We assume you are converting between grams C2H5OH and mole. A mole contains the same number of units by definition.
 Worksheet On Moles With Answer Key Docsity From docsity.com
Worksheet On Moles With Answer Key Docsity From docsity.com
This mass is given by the atomic weight of the chemical unit that makes up that substance in atomic mass units amu. A 602 x1023 atoms B 120 x 1023 atoms C 120 x 1022 atoms D 343 x 1023 atoms E 301 x 1024 atoms 1 mol 602 x 10. It means that if I have such a mass of silver there are Avogadros number N A 6022 1023 individual silver atoms. So moles of silver 145 g 10787 g mol1 00134 mol. Molecular weight of C2H5OH or mol The SI base unit for amount of substance is the mole. The SI base unit for amount of substance is the mole.
1 mole 60221023 6022 10 23 atoms molecules protons etc.
How many moles of silver are in the ring. Silver nitrate is the least expensive salt of silver. For instance if we wish to know the number of atoms in 025 moles of silver monatomic substance we just multiply Avogadros number and the number of moles. Answer 1 of 4. How many moles of silver are in the ring. LiClO3 1 mol LiClO3 172 X 1024 formula units KClO 3 Example 3 How many molecules are in 0944 moles of ammonia.
 Source: toppr.com
Source: toppr.com
We assume you are converting between grams Silver and mole. The SI base unit for amount of substance is the mole. Answer 1 of 4. One mole of silver atoms has a mass of 1079 g. Encyclopaedia Britannica states that there are 602000000000000000000000 or 602 followed by 21 zeroes atoms in a mole.
 Source: mammothmemory.net
Source: mammothmemory.net
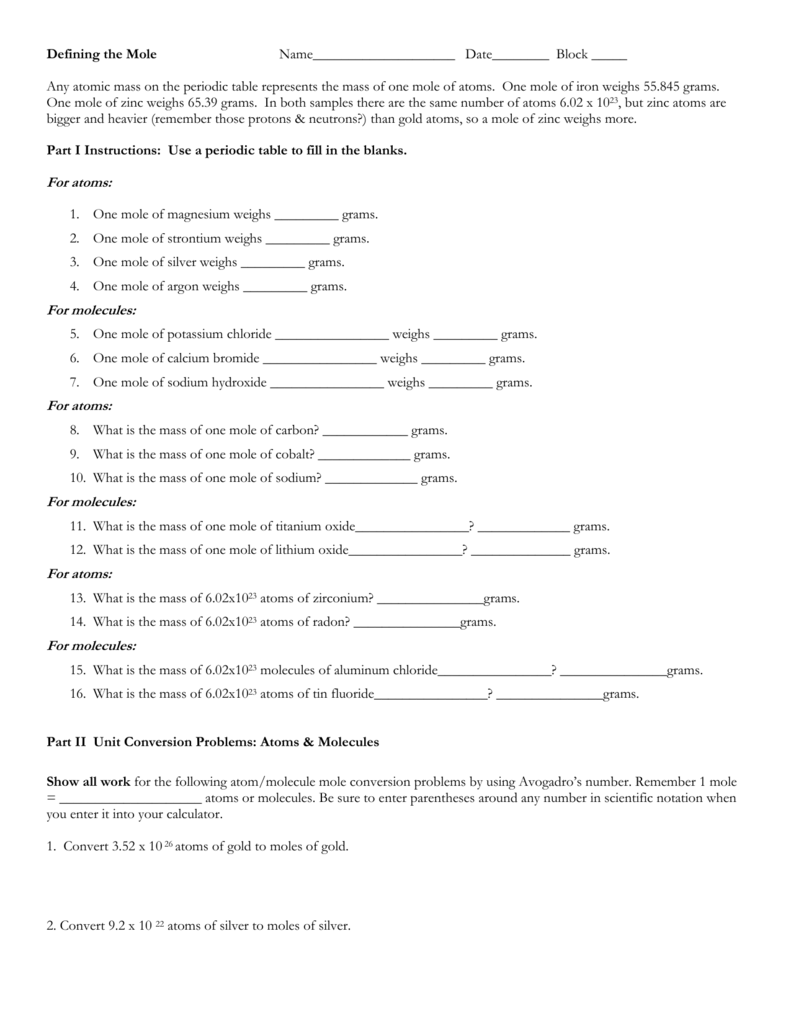
14 How many moles of silver are there in a pure sample containing 15 x 1023 atoms of silver. You can view more details on each measurement unit. How many moles of silver are in the ring. A 010 mol B 025 mol C 050 mol D 10 mol E 15 mol 1 mol 602 x 10 23 Slide 30 158 15 How many atoms are there in 500 mol of hafnium. You can view more details on each measurement unit.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
It means that if I have such a mass of silver there are Avogadros number N A 6022 1023 individual silver atoms. 0909 mol Ag 602 X 1023 atoms Ag 1 mol Ag 547 X 1023 atoms Ag Example 2 How many formula units are in 285 moles of lithium chlorate. The answer is 00092705727916105. The answer is 1078682. People found this article helpful.
 Source: slidetodoc.com
Source: slidetodoc.com
Then multiply n by the molar mass to get the answer. N moles of silver atoms will have a mass of n TIMES 1079 g. Encyclopaedia Britannica states that there are 602000000000000000000000 or 602 followed by 21 zeroes atoms in a mole. 0909 mol Ag 602 X 1023 atoms Ag 1 mol Ag 547 X 1023 atoms Ag Example 2 How many formula units are in 285 moles of lithium chlorate. How many moles of silver are in the ring.
 Source: docsity.com
Source: docsity.com
So all I have to do is divide the mass by the molar mass and multiply this number a molar quantity by Avogadros number. So 38 grams 3810782 moles. One 1 mole of silver contains 6022 x 1023 atoms Avogadros number One molar mass of Ag is 10782 grams. A mole contains the same number of units by definition. The answer is 4606844.
 Source: clutchprep.com
Source: clutchprep.com
1 mole 60221023 6022 10 23 atoms molecules protons etc. 245 mol Cu 6022 1023 atoms 1 mol 18 10 148 1024 atoms Cu 11 1022 atoms Ag 1 mol 6022 1023 atoms -2 mol Ag Molar Mass. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal. The molecular formula for Silver is Ag. How many moles of silver are in the ring.
 Source: studylib.net
Source: studylib.net
How many atoms does 1 mole of Si have. To convert moles to atoms. Then convert the moles. And the number of atoms 3810782 x Avogadros number 216 x 1022. 1 grams Silver is equal to 00092705727916105 mole.
 Source: slideplayer.com
Source: slideplayer.com
Answer 1 of 4. The answer is 00092705727916105. A 623 10 24 silver atoms B 226 10 24 silver atoms C 161 10 23 silver atoms D 244 10 26 silver atoms E 650 10 25 silver atoms Answer. A 010 mol B 025 mol C 050 mol D 10 mol E 15 mol 1 mol 602 x 10 23 Slide 30 158 15 How many atoms are there in 500 mol of hafnium. Silver nitrate is the least expensive salt of silver.
 Source: slideplayer.com
Source: slideplayer.com
This mass is given by the atomic weight of the chemical unit that makes up that substance in atomic mass units amu. Molecular weight of Silver or grams. Molecular weight of Silver or mol The molecular formula for Silver is Ag. 1 grams Silver is equal to 00092705727916105 mole. So all I have to do is divide the mass by the molar mass and multiply this number a molar quantity by Avogadros number.
 Source: studylib.net
Source: studylib.net
And number of silver atoms 00134 mol 6022 1023. Answer 1 of 4. The mass of 1 mol of atoms of an element An elements molar mass in gmol is numerically equal to the elements atomic mass in amu. One 1 mole of silver contains 6022 x 1023 atoms Avogadros number One molar mass of Ag is 10782 grams. The mole abbreviated mol is an SI unit which measures the number of particles in a specific substance.
 Source: studylib.net
Source: studylib.net
One mole is equal to 6022141791023 atoms or other elementary units such as molecules. And the number of atoms 3810782 x Avogadros number 216 x 1022. 1 mole 60221023 6022 10 23 atoms molecules protons etc. It means that if I have such a mass of silver there are Avogadros number N A 6022 1023 individual silver atoms. 1 Mole 60221415E23 Atom.
 Source: slideplayer.com
Source: slideplayer.com
This mass is given by the atomic weight of the chemical unit that makes up that substance in atomic mass units amu. For example silver has an atomic weight of 1078682 amu so one mole of silver has a mass of 1078682 grams. The answer is 4606844. 1 grams C2H5OH is equal to 0021706834440237 mole. The molar mass of silver is 1079 gmol.

How many atoms does 1 mole of Si have. 285 mol LiClO3 602 X 1023 fu. Atoms Cs 0404 mol Cs602 x 1023 atoms 243E23 atoms Csmol Multiply by atomsmole. People found this article helpful. A 010 mol B 025 mol C 050 mol D 10 mol E 15 mol 1 mol 602 x 10 23 Slide 30 158 15 How many atoms are there in 500 mol of hafnium.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
While the mass of 1 mole of a particular substance will vary 1 mole of ANY substance will ALWAYS have approximately 6021023 atoms rounded to 3 significant figures. The number of particles atoms molecules etc in one mole of anything is 60221023 Avogadros number. 23 x 1024 atoms Ag x 1 mole Ag 6022 x 1023 atoms Ag x 1070 g Ag 1 mole Ag 4121056 g Ag Convert the atoms Ag to moles of silver. One 1 mole of silver contains 6022 x 1023 atoms Avogadros number One molar mass of Ag is 10782 grams. How many atoms does 1 mole of Si have.
 Source: chegg.com
Source: chegg.com
Encyclopaedia Britannica states that there are 602000000000000000000000 or 602 followed by 21 zeroes atoms in a mole. 1 grams C2H5OH is equal to 0021706834440237 mole. 14 How many moles of silver are there in a pure sample containing 15 x 1023 atoms of silver. Is silver nitrate expensive. 285 mol LiClO3 602 X 1023 fu.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many atoms are in one mole of silver by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






