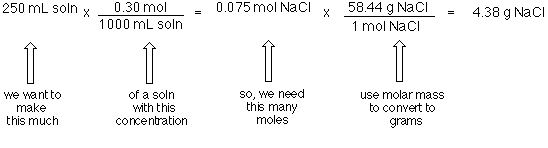Your How many atoms are in 1 mole of zinc images are available. How many atoms are in 1 mole of zinc are a topic that is being searched for and liked by netizens today. You can Get the How many atoms are in 1 mole of zinc files here. Find and Download all free vectors.
If you’re searching for how many atoms are in 1 mole of zinc images information related to the how many atoms are in 1 mole of zinc keyword, you have pay a visit to the right blog. Our site always provides you with hints for seeking the highest quality video and picture content, please kindly hunt and locate more informative video content and graphics that match your interests.
How Many Atoms Are In 1 Mole Of Zinc. How many atoms are in 1 mole of zinc. Molecular weight of Zinc or grams. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Oxygen or 159994 grams.
 Convert Liters To Cubic Meters 1 L Is 1000 Cm Cube Measurements Youtube Cube Converter Unit Conversion From pinterest.com
Convert Liters To Cubic Meters 1 L Is 1000 Cm Cube Measurements Youtube Cube Converter Unit Conversion From pinterest.com
This number is the number of atoms or. You can view more details on each measurement unit. 1 How many moles are 155 10²⁷ atoms of zinc. The number 6022 10²³ is called Avogadro number. Therefore 1 mole of zinc will weigh 65 grams. 1 000 000 000 000.
Mol-1 05 moles.
What number of moles are in 1 gram of zinc. The given problem will solve by using Avogadro number. 65 moles 602 x 1023 atoms 39 x 1024 atoms 1 mole 2. For the rest of the atoms on the periodic table their molar mass tells you how many grams of each you need to have a mole of those atoms or 6021023 atoms of that element. 24 x 1024 atoms of argon 1 mole 40 mol 602 x 1023 atoms 3. A mole is simple a convenient way of estimating the number particles within a substance.
 Source: pinterest.com
Source: pinterest.com
Density of zinc is equal to 7 133 kgm³. So the total charge of 1 mole zinc is 0. Molecular weight of Zinc or grams. 1 How many moles are 155 10²⁷ atoms of zinc. Therefore 1 mole of zinc will weigh 65 grams.
 Source: pinterest.com
Source: pinterest.com
1 mole 602 x 1023 atoms 1 mole atomic mass g Try. Simply so how many atoms are in 750 moles of zinc. 1 grams Zinc is equal to 0015295197308045 mole. Thus the total number of moles in 327 grams of zinc 327g65g. Molecular weight of Zinc or mol The molecular formula for Zinc is Zn.
 Source: fr.pinterest.com
Source: fr.pinterest.com
One mole of zinc will have 6022 x 1023 zinc atoms and so forth. Since we know that there are 60221023 atoms in every mole of a substance Avogadros Number there are 6022E230750 atoms of Zn in 0750 mols of Zn. How many moles of atoms are contained in 225 g of zinc. The SI base unit for amount of substance is the mole. The number of moles present in 1 mole of zinc will be 602 x 10²³ atoms.
 Source: pinterest.com
Source: pinterest.com
How many atoms are in 1 mole of zinc. It is the number of atoms ions and molecules in one gram atom of element one gram molecules of compound and one gram ions of a substance. The SI base unit for amount of substance is the mole. For the rest of the atoms on the periodic table their molar mass tells you how many grams of each you need to have a mole of those atoms or 6021023 atoms of that element. The answer is 0015295197308045.
 Source: pinterest.com
Source: pinterest.com
At 25C 77F or 29815K at standard atmospheric pressure. 24 x 1024 atoms of argon 1 mole 40 mol 602 x 1023 atoms 3. This is because a mole of any substance contains the Avogadros number of particles which is 602 x 10²³ atoms. Moles n mass mmolar mass M. 1 mole N A.
 Source: pinterest.com
Source: pinterest.com
The number 6022 10²³ is called Avogadro number. 1 grams Zinc is equal to 0015295197308045 mole. The answer is 0015295197308045. How many moles of atoms are contained in 225 g of zinc. 1 mole 602 x 1023 atoms 1 mole atomic mass g Try.
 Source: pinterest.com
Source: pinterest.com
What number of moles are in 1 gram of zinc. One mole of zinc will have 6022 x 1023 zinc atoms and so forth. Avogadros Number 6022xx1023mol-1. I used Avogadros Number. What number of moles are in 1 gram of zinc.
 Source: pinterest.com
Source: pinterest.com
If zinc has 1 mole it will have 6021023. There are 6022 x 1023 particles in 1 mole of zinc. What number of moles are in 1 gram of zinc. 1 mole is equal to 1 moles Oxygen or 159994 grams. 24 x 1024 atoms of argon 1 mole 40 mol 602 x 1023 atoms 3.
 Source: in.pinterest.com
Source: in.pinterest.com
The SI base unit for amount of substance is the mole. Simply so how many atoms are in 750 moles of zinc. Molecular weight of Zinc or mol The molecular formula for Zinc is Zn. We assume you are converting between moles Zinc and gram. How many atoms are in 65 moles of zinc.
 Source: pinterest.com
Source: pinterest.com
3 moles of Zn is equal to 1809x1023 atoms. Avogadros Number 6022xx1023mol-1. Since we know that there are 60221023 atoms in every mole of a substance Avogadros Number there are 6022E230750 atoms of Zn in 0750 mols of Zn. How many atoms are in 65 moles of zinc. The SI base unit for amount of substance is the mole.
 Source: pinterest.com
Source: pinterest.com
First a bit about Avogadros number. 1 mole is the same as 1 moles Zinc or 6538 grams. 3 moles of Zn is equal to 1809x1023 atoms. Zinc weighs 7133 gram per cubic centimeter or 7 133 kilogram per cubic meter ie. The given problem will solve by using Avogadro number.
 Source: pinterest.com
Source: pinterest.com
Simply so how many atoms are in 750 moles of zinc. So you have 175xxN_A atoms of zinc. See 1 mole zinc consists of 6021023 zinc atoms. 1 10 -6. The SI base unit for amount of substance is the mole.
 Source: pinterest.com
Source: pinterest.com
Use this page to learn how to convert between grams Zinc and mole. Click hereto get an answer to your question How many atoms are in 1 mole of zinc. The molar mass of zinc is 65 grams per mole. At 25C 77F or 29815K at standard atmospheric pressure. There are 6022 x 1023 particles in 1 mole of zinc.
 Source: pinterest.com
Source: pinterest.com
How many atoms are in 1 mole of zinc. It is the number of atoms ions and molecules in one gram atom of element one gram molecules of compound and one gram ions of a substance. A mole is simple a convenient way of estimating the number particles within a substance. The SI base unit for amount of substance is the mole. Avogadros Number is the calculated value of the number of atoms molecules etc.
 Source: pinterest.com
Source: pinterest.com
The number of atoms in 1 mole of Zinc is equal to 60221023atoms 6022 10 23 a t o m s. 1 10 -6. First a bit about Avogadros number. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Oxygen or 159994 grams.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many atoms are in 1 mole of zinc by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.