Your How many atoms are in 1 mole of silver images are available in this site. How many atoms are in 1 mole of silver are a topic that is being searched for and liked by netizens today. You can Get the How many atoms are in 1 mole of silver files here. Download all free vectors.
If you’re looking for how many atoms are in 1 mole of silver images information connected with to the how many atoms are in 1 mole of silver keyword, you have come to the right site. Our site always gives you suggestions for seeing the maximum quality video and image content, please kindly search and locate more enlightening video articles and graphics that match your interests.
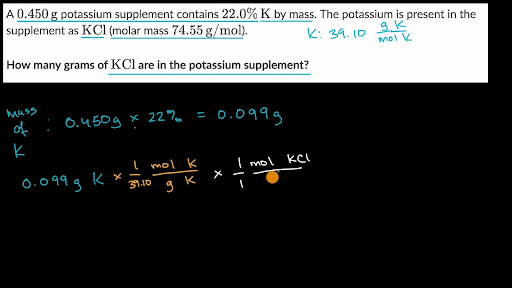
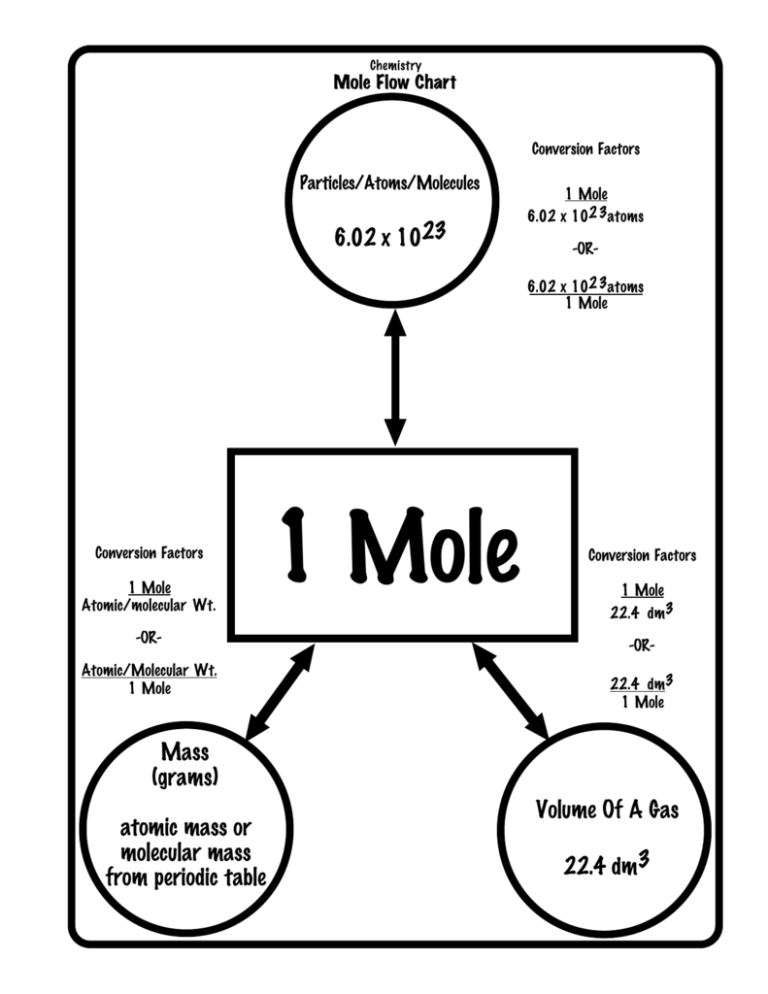
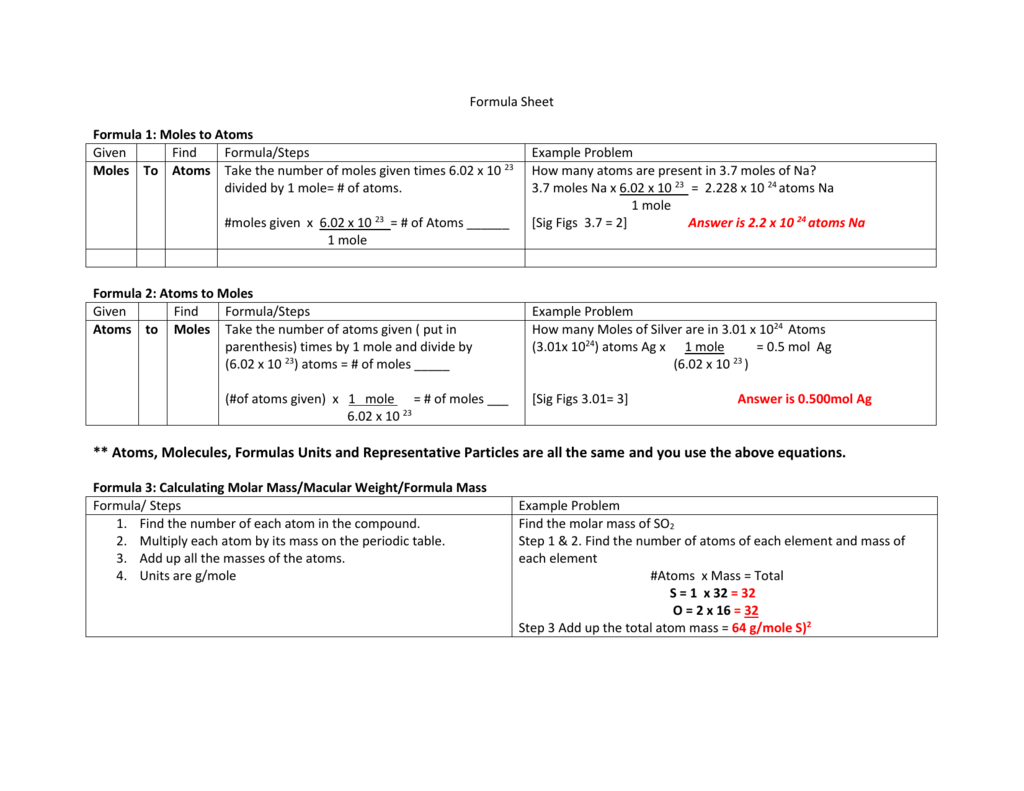
How Many Atoms Are In 1 Mole Of Silver. 0909 mol Ag 602 X 1023 atoms Ag 1 mol Ag 547 X 1023 atoms Ag Example 2 How many formula units are in 285 moles of lithium chlorate. A silver ring contains 11 x 1022 silver atoms. You can view more details on each measurement unit. 23 atoms 1 mol or 1 mol 6022 1023 atoms Converting between Number of Moles and Number of Atoms Calculate the number of atoms in 245 mol of copper.
 Chapter 10 Answers Ppt Download From slideplayer.com
Chapter 10 Answers Ppt Download From slideplayer.com
623 x 10 24 silver atoms. 1 grams Silver is equal to 00092705727916105 mole. 65 How many silver atoms are contained in 375 moles of silver. A silver ring contains 11 x 1022 silver atoms. The mole is thus the link between the micro world of atoms and molecules to the macro world of grams and litres. We assume you are converting between grams Silver and mole.
How many moles of silver are in the ring.
A 010 mol B 025 mol C 050 mol D 10 mol E 15 mol 1 mol 602 x 10 23 Slide 30 158 15 How many atoms are there in 500 mol of hafnium. 285 mol LiClO3 602 X 1023 fu. How many moles of silver are in the ring. 1 mole of silver has 6022 x 1023 atoms of silver Therefore 567 moles of silver has 567 x 6022 x 1023 atoms of silver 341 x 1024 atoms of silver to 2 significant figures. So moles of silver 145 g 10787 g mol1 00134 mol. How many atoms are in 100g of gold.
 Source: studylib.net
Source: studylib.net
The answer is 1078682. One 1 mole of silver contains 6022 x 1023 atoms Avogadros number One molar mass of Ag is 10782 grams. 285 mol LiClO3 602 X 1023 fu. 14 How many moles of silver are there in a pure sample containing 15 x 1023 atoms of silver. The molecular formula for Silver is Ag.
 Source: slideplayer.com
Source: slideplayer.com
We assume you are converting between grams Silver and mole. The answer is 1078682. A 010 mol B 025 mol C 050 mol D 10 mol E 15 mol 1 mol 602 x 10 23 Slide 30 158 15 How many atoms are there in 500 mol of hafnium. How many moles are equal to 625g of copper. A 623 10 24 silver atoms B 226 10 24 silver atoms C 161 10 23 silver atoms D 244 10 26 silver atoms E 650 10 25 silver atoms Answer.
 Source: studylib.net
Source: studylib.net
Is silver nitrate expensive. 65 How many silver atoms are contained in 375 moles of silver. A 623 10 24 silver atoms B 226 10 24 silver atoms C 161 10 23 silver atoms D 244 10 26 silver atoms E 650 10 25 silver atoms Answer. 226 x 10 24 silver atoms. How many atoms are in 100g of gold.
 Source: slideplayer.com
Source: slideplayer.com
0909 mol Ag 602 X 1023 atoms Ag 1 mol Ag 547 X 1023 atoms Ag Example 2 How many formula units are in 285 moles of lithium chlorate. Now 1 mole of silver atoms or in a 10787g mass there are by definition 60221023 individual atoms of silver. So moles of silver 145 g 10787 g mol1 00134 mol. The molecular formula for Silver is Ag. 226 x 10 24 silver atoms.
 Source: docplayer.net
Source: docplayer.net
623 x 10 24 silver atoms. And the number of atoms 3810782 x Avogadros number 216 x 1022. A 602 x1023 atoms B 120 x 1023 atoms C 120 x 1022 atoms D 343 x 1023 atoms E 301 x 1024 atoms 1 mol 602. 1 grams Silver is equal to 00092705727916105 mole. You can view more details on each measurement unit.
 Source: clutchprep.com
Source: clutchprep.com
Is silver nitrate expensive. The SI base unit for amount of substance is the mole. The molecular formula for Silver is Ag. And the number of atoms 3810782 x Avogadros number 216 x 1022. Molecular weight of Silver or mol.
 Source: studylib.net
Source: studylib.net
How many moles are equal to 625g of copper. 1 mole of silver has 6022 x 1023 atoms of silver Therefore 567 moles of silver has 567 x 6022 x 1023 atoms of silver 341 x 1024 atoms of silver to 2 significant figures. The answer is 00092705727916105. This number can be atoms molecules ions etc. 14 How many moles of silver are there in a pure sample containing 15 x 1023 atoms of silver.
 Source: studylib.net
Source: studylib.net
Molecular weight of Silver or grams. 225 x 1025 atoms of lead 1 mole 2072g 774419g 7740g 602 x 1023 atoms 1 mole 2. We assume you are converting between grams Silver and mole. The molecular formula for Silver is Ag. The molecular formula for Silver is Ag.
 Source: studylib.net
Source: studylib.net
So all I have to do is divide the mass by the molar mass and multiply this number a molar quantity by Avogadros number. A mole is also related to atomic weight or mass. What is the mass of 1 atom of silver in grams. You can view more details on each measurement unit. For example one mole of carbon-12 atoms has an atomic weight of 12 grams while a mole of oxygen which also contains Avogadros number of units has an atomic weight of 16 grams.
 Source: mammothmemory.net
Source: mammothmemory.net
The answer is 1078682. The molecular formula for Silver is Ag. May 23 2012. How many atoms are in 100g of gold. 625g of copper 1 mol 977 mol Cu 64 g Cu 2.
 Source: studylib.net
Source: studylib.net
Recall that one mole of any substance is equal to Avogadros number which is. A 602 x1023 atoms B 120 x 1023 atoms C 120 x 1022 atoms D 343 x 1023 atoms E 301 x 1024 atoms 1 mol 602. 23 atoms 1 mol or 1 mol 6022 1023 atoms Converting between Number of Moles and Number of Atoms Calculate the number of atoms in 245 mol of copper. 225 x 1025 atoms of lead 1 mole 2072g 774419g 7740g 602 x 1023 atoms 1 mole 2. A silver ring contains 11 x 1022 silver atoms.

The mole is thus the link between the micro world of atoms and molecules to the macro world of grams and litres. 226 x 10 24 silver atoms. You can view more details on each measurement unit. Recall that one mole of any substance is equal to Avogadros number which is. A 602 x1023 atoms B 120 x 1023 atoms C 120 x 1022 atoms D 343 x 1023 atoms E 301 x 1024 atoms 1 mol 602.
 Source: slideplayer.com
Source: slideplayer.com
The answer is 00092705727916105. Molecular weight of Silver or grams. How many moles are equal to 625g of copper. The molecular formula for Silver is Ag. The molecular formula for Silver is Ag.
 Source: slidetodoc.com
Source: slidetodoc.com
The molecular formula for Silver is Ag. The answer is 1078682. 344 x 10 23 silver atoms. 245 mol Cu 6022 1023 atoms 1 mol 18 10 148 1024 atoms Cu 11 1022 atoms Ag 1 mol 6022 1023 atoms -2 mol Ag. How many moles are equal to 625g of copper.
 Source: slideplayer.com
Source: slideplayer.com
We assume you are converting between grams Silver and mole. The answer is 00092705727916105. How many moles of silver are in the ring. 226 x 10 24 silver atoms. How many moles of silver atoms are in 18 Ã 1020 atoms of silver.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many atoms are in 1 mole of silver by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.