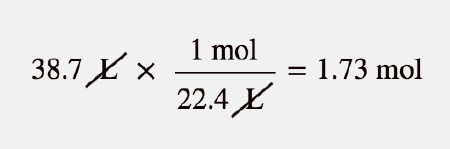Your How many atoms are in 1 mole of hydrogen gas images are ready. How many atoms are in 1 mole of hydrogen gas are a topic that is being searched for and liked by netizens today. You can Download the How many atoms are in 1 mole of hydrogen gas files here. Download all free images.
If you’re searching for how many atoms are in 1 mole of hydrogen gas pictures information linked to the how many atoms are in 1 mole of hydrogen gas topic, you have visit the ideal site. Our site always provides you with suggestions for downloading the highest quality video and picture content, please kindly hunt and find more enlightening video content and images that fit your interests.
How Many Atoms Are In 1 Mole Of Hydrogen Gas. Now number of moles of CH 4 in 52 1024 molecules is. To convert from moles to atoms multiply the molar amount by Avogadros number. Since we have 2 moles of hydrogen gas the number of molecules of hydrogen present are. This quantity is equivalent to.
 How Many Molecules Are Present In One In One Gram Of Hydrogen Youtube From youtube.com
How Many Molecules Are Present In One In One Gram Of Hydrogen Youtube From youtube.com
Avogadros law specifies that the volume of one mole of any gas at STP is 224 L. Molecular weight of Hydrogen or grams. 1 water molecule 2 Hydrogen atoms 1 oxygen atom. This quantity is equivalent to. To convert from moles to atoms multiply the molar amount by Avogadros number. In every molecule there are two atoms so the number of atoms in 1 mol of hydrogen would be 6021023212041023 atoms.
In every molecule there are two atoms so the number of atoms in 1 mol of hydrogen would be 6021023212041023 atoms.
602 10 23. In one mole of. Moles of NH42SO4 N H 4 2 S O 4 020mol. Avogadros number is a very important relationship to remember. It consists of one atom. Note that rounding errors may occur so always check the results.
 Source: pinterest.com
Source: pinterest.com
Contains two atoms of hydrogen. In one mole of. 1 water molecule 2 Hydrogen atoms 1 oxygen atom. The molecular formula of methane CH 4 suggests that one molecule of this compound consists of 4 atoms of H. In one mole of Hydrogen gasthere would be6.

Each molecule of hydrogen gas H2 is made of two hydrogen atoms. Avogadros number is a very important relationship to remember. A mole is Avogadros number of particles which. So the number of moles of H atom in it. 1 grams Hydrogen is equal to 0992122546977 mole.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
The SI base unit for amount of substance is the mole. In every molecule there are two atoms so the number of atoms in 1 mol of hydrogen would be 6021023212041023 atoms. But since each individual molecule consists of 2 atoms of nitrogen the number of moles of nitrogen atoms will be twice that of nitrogen gas moleculesAlternatively you can express this as 2NA where NA is Avogadros constant. Contains two atoms of hydrogen. Now the number of atomsmolecules present in 1 mole of a substance is represented by a quantity known as Avogadros Number.

1 mole 60221023 6022 10 23 atoms molecules protons etc. Contains two atoms of hydrogen. Mar 13 2017. 4 52 1024 Avogadros number. 1 grams Hydrogen is equal to 0992122546977 mole.
 Source: youtube.com
Source: youtube.com
1 gm of hydrogen contains 2160210 23. Since water has a chemical formula of H2O there will be 2 moles of hydrogen in every mole of water. Therefore 2 mole H2O will have 240881024 hydrogen atoms. The molecular formula for Hydrogen is H. 1 mole of hydrogen gas contains 60210 23 atoms.
 Source: youtube.com
Source: youtube.com
The mole mol is a unit of measure for an amount. A water particle is made up of 3atoms A mole of water is 18 grams 16 of those grams are oxygen atoms as well as 2 grams are hydrogens. 1008 gramsmole Hydrogen 1 mole6022x1023 atoms 167 x 10-24 grams. Avogadros number is a very important relationship to remember. 4 52 1024 Avogadros number.
 Source: slideplayer.com
Source: slideplayer.com
602 1023carbon atoms. Also know how many hydrogen atoms are there in 300 mole of h2o. So 1 litre is 1224 moles of hydrogen gas. Of a chemical substance. Likewise people ask how many atoms are in a mole.
 Source: pinterest.com
Source: pinterest.com
So the requires weight is 2224 grams. 1 mole 60221023 6022 10 23 atoms molecules protons etc. In every molecule there are two atoms so the number of atoms in 1 mol of hydrogen would be 6021023212041023 atoms. So 1 litre is 1224 moles of hydrogen gas. The molecular formula for Hydrogen is H.
 Source: slideplayer.com
Source: slideplayer.com
Type in your own numbers in the form to convert the units. Also know how many hydrogen atoms are there in 300 mole of h2o. 52 1024 Avogadros number. Mar 13 2017. 1 water molecule 2 Hydrogen atoms 1 oxygen atom.
 Source: sk.pinterest.com
Source: sk.pinterest.com
This quantity is equivalent to. 1 mole 60221023 6022 10 23 atoms molecules protons etc. The molecular formula of methane CH 4 suggests that one molecule of this compound consists of 4 atoms of H. So 1 litre is 1224 moles of hydrogen gas. These numbers are either in atomic mass units amu or in grams per mole of atoms.

But since each individual molecule consists of 2 atoms of nitrogen the number of moles of nitrogen atoms will be twice that of nitrogen gas moleculesAlternatively you can express this as 2NA where NA is Avogadros constant. 52 1024 Avogadros number. In every molecule there are two atoms so the number of atoms in 1 mol of hydrogen would be 6021023212041023 atoms. Of a chemical substance. Therefore 2 mole H2O will have 240881024 hydrogen atoms.
 Source: youtube.com
Source: youtube.com
Likewise people ask how many atoms are in a mole. So 1 mole H2O 120441024 hydrogen atoms. Therefore 2 mole H2O will have 240881024 hydrogen atoms. There are 2 atoms of hydrogen as well as one atom of oxygen in each water particle making the formula H 2 O. A water particle is made up of 3atoms A mole of water is 18 grams 16 of those grams are oxygen atoms as well as 2 grams are hydrogens.
 Source: pinterest.com
Source: pinterest.com
So 1 litre is 1224 moles of hydrogen gas. How many moles of hydrogen gas are present in liters at STP. Of a chemical substance. The equality of 1 mole 224 L is the basis for the conversion factor. That said to find the mass of one ATOM we need to convert from moles to atoms as follows.
 Source: youtube.com
Source: youtube.com
In one mole of Hydrogen gasthere would be6021023molecules. In one mole of. Avogadros number is a very important relationship to remember. Accordingly how many hydrogen atoms are there in 300 mole of h2o. In one mole of Hydrogen gasthere would be6.
 Source: pinterest.com
Source: pinterest.com
So the number of moles of H atom in it. Atoms per mole of a substance. A mole is Avogadros number of particles which. In every molecule there are two atoms so the number of atoms in 1 mol of hydrogen would be 6021023212041023 atoms. This means that 1 MOLE of hydrogen atoms will weigh 1008 grams.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many atoms are in 1 mole of hydrogen gas by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.