Your How many atoms are in 1 mole of h2so4 images are available in this site. How many atoms are in 1 mole of h2so4 are a topic that is being searched for and liked by netizens today. You can Get the How many atoms are in 1 mole of h2so4 files here. Download all free images.
If you’re searching for how many atoms are in 1 mole of h2so4 pictures information connected with to the how many atoms are in 1 mole of h2so4 topic, you have visit the right site. Our website always gives you hints for downloading the maximum quality video and image content, please kindly surf and locate more enlightening video articles and images that match your interests.
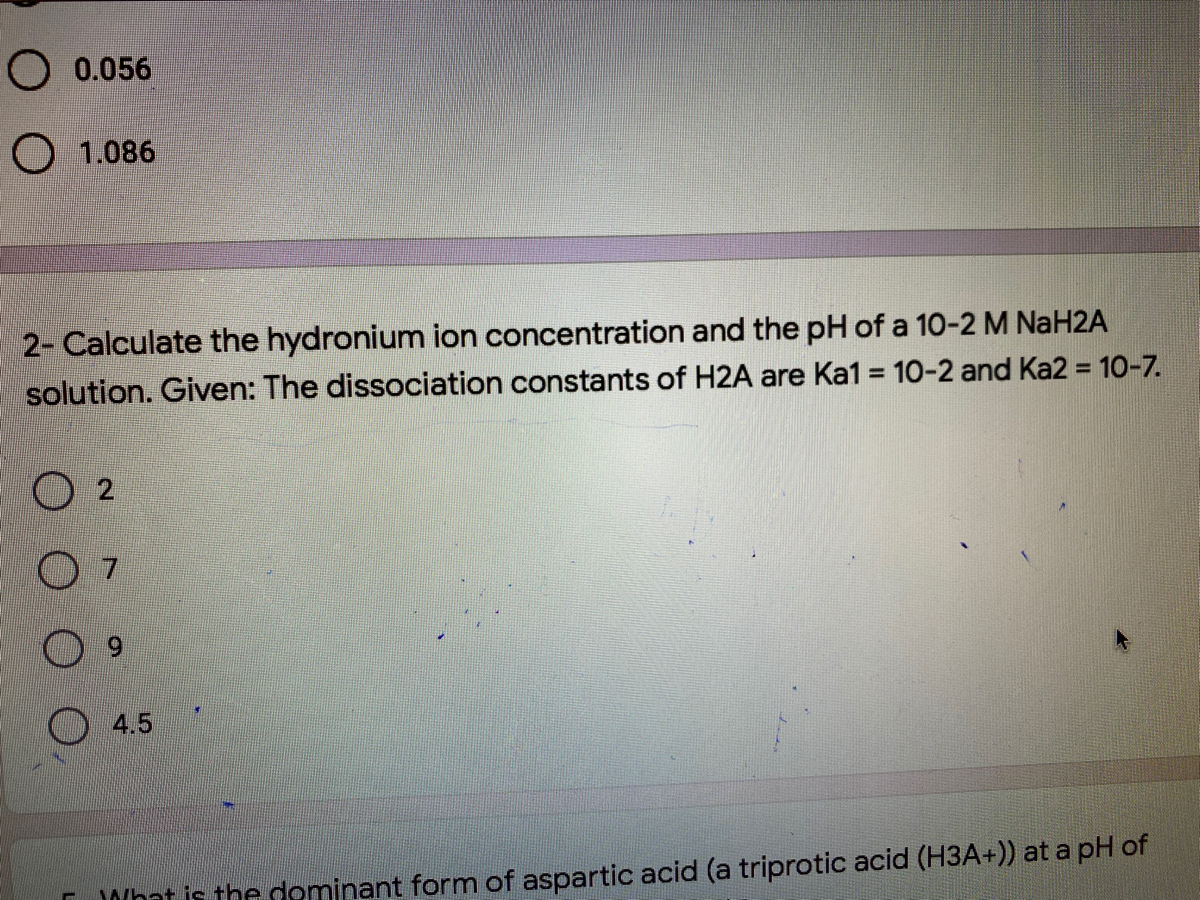
How Many Atoms Are In 1 Mole Of H2so4. 0 2 1 0 2 3. How many moles are present in one mole of H2SO4. 1007942 32065 1599944 Percent composition by element. Exothermic because heat is released.
 The Following Questions Are About One Mole Of Sulphuric Acid H2so4 Nu A Find The Number Of Gram Hydrogen Atoms In 1 Mole Of H2so41 B How Many Atoms Of Hydrogen Does From toppr.com
The Following Questions Are About One Mole Of Sulphuric Acid H2so4 Nu A Find The Number Of Gram Hydrogen Atoms In 1 Mole Of H2so41 B How Many Atoms Of Hydrogen Does From toppr.com
0 2 1 0 2 3. Convert grams H2SO4 to moles or moles H2SO4 to grams. So 1 mole of H2SO4 has4 x N atoms of oxygen. You can also say one mole of sulfuric acid has two moles of hydrogen atoms 1 mole of sulfur atoms and 4 moles of oxygen atoms. Click hereto get an answer to your question How many gram atoms of sulfur are there in 49 g of H2SO4 and of nitrogen in 126 g of HNO3 Atomic weights of H 1 N 14 O 16 S 32. 0 2 1 0 2 3.
One sulfuric H2SO4 molecule has 2 hydrogen atoms 1 sulfur atom and 4 oxygen atoms.
This compound is also known as Sulfuric Acid. 30581 x 6022 1023 184x1024. H2SO4 molecular weight. Atomic mass of one mole of H2SO4 is 98 grams. Atomic masses can be located on most periodic tables of elements and may vary slightly from table to table. Correct option is.
 Source: toppr.com
Source: toppr.com
Now we just punch in the numbers and find. As the molecule this is as far as you go. 1007942 32065 1599944 Percent composition by element. In one H2SO4 there are 7 atoms. Atomic mass of one mole of H2SO4 is 98 grams.
 Source: toppr.com
Source: toppr.com
You can also say one mole of sulfuric acid has two mols of hydrogen atoms 1 mol of sulfur atoms and 4 moles of oxygen atoms. One sulfuric H2SO4 molecule has 2 hydrogen atoms 1 sulfur atom and 4 oxygen atoms. Next multiply avogadros constant. The molar mass of H2SO4 is 98 gramsmol so in 98g of sulfuric acid there is exactly 1 mole of sulfuric acid. 05 mole of H2SO4 has05 x 4 x N 05 x 4 x 6023 x 1023 atoms of oxygen 2 x 6022 x 1023 12044 x10 24 atoms of oxygen.
 Source: instasolv.com
Source: instasolv.com
0 2 1 0 2 3. Since a single molecule of H2SO4 contains 2 atoms of Hydrogen 1 of Sulphur and 4 of Oxygen and one mole contains 602 x 1023 molecules then there are 4 x 602 x 1023 atoms of Oxygen which is. You can also say one mole of sulfuric acid has two moles of hydrogen atoms 1 mole of sulfur atoms and 4 moles of oxygen atoms. So 1 mole of H2SO4 has4 x N atoms of oxygen. 1007942 32065 1599944 Percent composition by element.
 Source: youtube.com
Source: youtube.com
One sulfuric H2SO4 molecule has 2 hydrogen atoms 1 sulfur atom and 4 oxygen atoms. Since youre dealing with one mole of sulfuric acid it follows that you will also be dealing with two moles of hydrogen. 30581 x 6022 1023 184x1024. Now we can do the same for the other two compounds. You can also say one mole of sulfuric acid has two moles of hydrogen atoms 1 mole of sulfur atoms and 4 moles of oxygen atoms.
 Source: pinterest.com
Source: pinterest.com
There are seven atoms in one molecule of the compound H2SO4. Since youre dealing with one mole of sulfuric acid it follows that you will also be dealing with two moles of hydrogen. Click hereto get an answer to your question How many gram atoms of sulfur are there in 49 g of H2SO4 and of nitrogen in 126 g of HNO3 Atomic weights of H 1 N 14 O 16 S 32. This compound is also known as Sulfuric Acid. As the molecule this is as far as you go.
 Source: toppr.com
Source: toppr.com
Was this answer helpful. How many moles of H are in H2SO4. How many atoms are present in 1 mole of H2SO4. This means that every mole of sulfuric acid has a mass of 980795 g. Was this answer helpful.

30581 x 6022 1023 184x1024. Was this answer helpful. So 1 mole of H2SO4 has4 x N atoms of oxygen. How many atoms are present in 1 mole of H2SO4. You can also say one mole of sulfuric acid has two moles of hydrogen atoms 1 mole of sulfur atoms and 4 moles of oxygen atoms.

This means that one mole of sulfuric acid will contain 2 moles of hydrogen atoms. One sulfuric H2SO4 molecule has 2 hydrogen atoms 1 sulfur atom and 4 oxygen atoms. 0 2 1 0 2 3. Correct option is. Consider the following reaction.
 Source: youtube.com
Source: youtube.com
0 2 1 0 2 3. This means that one mole of sulfuric acid will contain 2 moles of hydrogen atoms. Exothermic because heat is released. Now if you dissolve this into water dilute enough that it fully dissociates then you will get 2 moles of H. 0 2 1 0 2 3.
 Source: bartleby.com
Source: bartleby.com
In one H2SO4 there are 7 atoms. Molecular Formula of Sulphuric acid H2SO4 The subscript present with element gives its number of atoms in its mloecule Number of atoms of Hydrogen in one mole of sulphuric acid 26023102312051024 Number of atoms of sulphur in one mole of sulphuric acid 16023102360231023 Number of atoms of oxygen in one mole of sulphuric acid. Atomic mass of one mole of H2SO4 is 98 grams. How many atoms are present in 1 mole of Sulphuric acid. Atomic masses can be located on most periodic tables of elements and may vary slightly from table to table.
 Source: toppr.com
Source: toppr.com
You can also say one mole of sulfuric acid has two moles of hydrogen atoms 1 mole of sulfur atoms and 4 moles of oxygen atoms. Exothermic because heat is released. 1 molcule of H2SO4 has4 atoms of Oxygen. Correct option is. One sulfuric H2SO4 molecule has 2 hydrogen atoms 1 sulfur atom and 4 oxygen atoms.

Was this answer helpful. Advertisement Advertisement New questions in Chemistry. How many atoms are present in mole of H 2 S O 4. 05 mole of H2SO4 has05 x 4 x N 05 x 4 x 6023 x 1023 atoms of oxygen 2 x 6022 x 1023 12044 x10 24 atoms of oxygen. Since youre dealing with one mole of sulfuric acid it follows that you will also be dealing with two moles of hydrogen.
 Source: slideplayer.com
Source: slideplayer.com
Chemistry University of Wisconsin-Madison 1982. Was this answer helpful. How many moles are present in one mole of H2SO4. As the molecule this is as far as you go. Atomic mass of one mole of H2SO4 is 98 grams.
 Source: chegg.com
Source: chegg.com
This means that every mole of sulfuric acid has a mass of 980795 g. How many atoms are present in mole of H 2 S O 4. 0 2 1 0 2 3. Convert grams H2SO4 to moles or moles H2SO4 to grams. H2SO4 molecular weight.

For every mole of H2SO4 molecules there are 4 moles of oxygen atoms which has a molar mass of 1600 gmol mass of the part. Correct option is. How many moles of H are in H2SO4. Atomic masses can be located on most periodic tables of elements and may vary slightly from table to table. Chemistry University of Wisconsin-Madison 1982.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many atoms are in 1 mole of h2so4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






