Your How many atoms are in 1 mole of h2o images are available. How many atoms are in 1 mole of h2o are a topic that is being searched for and liked by netizens now. You can Find and Download the How many atoms are in 1 mole of h2o files here. Get all free photos.
If you’re searching for how many atoms are in 1 mole of h2o pictures information linked to the how many atoms are in 1 mole of h2o interest, you have come to the right site. Our website frequently provides you with suggestions for seeking the maximum quality video and picture content, please kindly surf and find more enlightening video content and images that fit your interests.
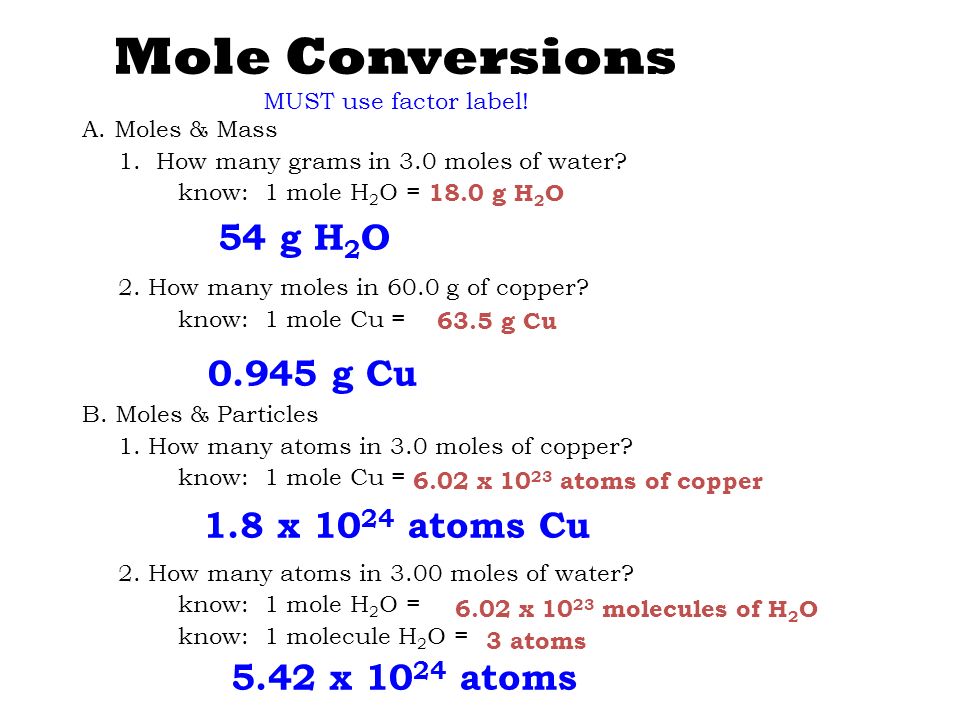
How Many Atoms Are In 1 Mole Of H2o. Mass of 1 Mole of Water Water H2O is made from 2 atoms of hydrogen and 1 atom of oxygen. Molecular weight of H2O or mol This compound is also known as Water or Dihydrogen Monoxide. So 1 mole H2O 120441024 hydrogen atoms. Determine the molar mass of water.
 The Mole Chemistry Ppt Download From slideplayer.com
The Mole Chemistry Ppt Download From slideplayer.com
Also know how many hydrogen atoms are there in 300 mole of h2o. How many grams H2O in 1 mol. 10 g gold 1 mole 602 x 1023 atoms 306 x 1022 atoms 1970g 1 mole PRACTICE PROBLEMS. One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 10 23 atoms of oxygen. In one mole of water there will exist approximately 6021023 water molecules. Since water has a chemical formula of H2O there will be 2 moles of hydrogen in every mole of water.
And since the chemical formula for water is H 2O 1 mole of water corresponds to 1 mole of oxygen atoms.
So to get number of atoms in 18g of water we will multiply Avogadros number by 3. In this manner how many atoms are in 2 moles of h2o. As 6022 10²³ number of hydrogen atoms present in 1 mole So 129 10²⁴ number of hydrogen atoms present in mole Part 1b. If you have a compound like H 2 O then. 225 x 1025 atoms of lead 1 mole 2072g 774419g 7740g 602 x 1023 atoms 1 mole 2. 736 10²⁴ oxygen atoms.
 Source: amburnclasses.org
Source: amburnclasses.org
As 6022 10²³ number of hydrogen atoms present in 1 mole So 129 10²⁴ number of hydrogen atoms present in mole Part 1b. How many grams H2O in 1 mol. Likewise how many oxygen atoms are there in 18 gram of water. You can view more details on each measurement unit. In one mole of water there will exist approximately 6021023 water molecules.
 Source: slideplayer.com
Source: slideplayer.com
As 6022 10²³ number of hydrogen atoms present in 1 mole So 129 10²⁴ number of hydrogen atoms present in mole Part 1b. So 1 mole H2O 120441024 hydrogen atoms. 736 10²⁴ oxygen atoms. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. The SI base unit for amount of substance is the mole.
 Source: slidetodoc.com
Source: slidetodoc.com
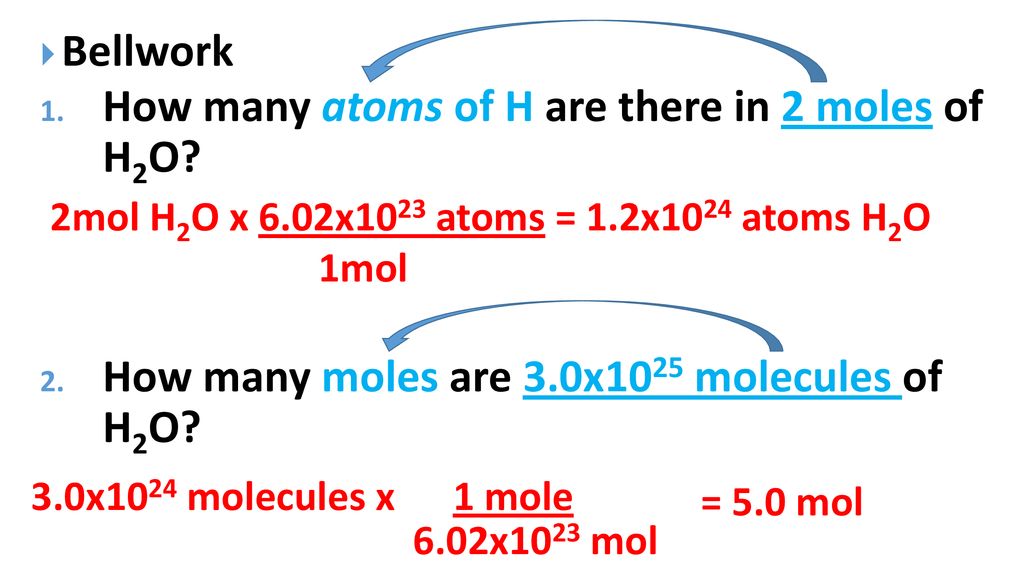
Likewise in order to get 6022 x 1023 molecules of H2O a mole of water we need 12044 x 1023 which is 6022 x 1023 x 2 atoms of hydrogen and 6022 x 1023 atoms of oxygen. 10 g gold 1 mole 602 x 1023 atoms 306 x 1022 atoms 1970g 1 mole PRACTICE PROBLEMS. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. So 1 mole H2O 120441024 hydrogen atoms. If we total up the gram amounts of each element in the water molecule 15998gmol 21008gmol we get the molar mass of water 18014gmol.

In this manner how many atoms are in 2 moles of h2o. The answer is 1640878. If you have a compound like H 2 O then. Since water is H2O one molecule has 3 atoms 2 of hydrogen1 of oxygenSo total atoms 360231023 180691023 atoms. In one mole of water there will exist approximately 602 1023 water molecules.
 Source: slideplayer.com
Source: slideplayer.com
You can view more details on each measurement unit. But each molecule of water contains 2 H and 1 O atom 3 atoms so there are approximately 18 x 10 24 atoms in a mole of water. A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms. We assume you are converting between grams H2O and mole. The mass of hydrogen is 1008 gmol and the mass of oxygen is 1600 gmol so the mass of a mole of water can be calculated as follows.

Therefore 2 mole H2O will have 240881024 hydrogen atoms. And since the chemical formula for water is H 2O 1 mole of water corresponds to 1 mole of oxygen atoms. So 1 mole H2O 120441024 hydrogen atoms. Click to see full answer. Also know how many hydrogen atoms are there in 300 mole of h2o.
 Source: slideplayer.com
Source: slideplayer.com
10 g gold 1 mole 602 x 1023 atoms 306 x 1022 atoms 1970g 1 mole PRACTICE PROBLEMS. How many grams H2O in 1 mol. So 1 mole H2O 120441024 hydrogen atoms. 1 water molecule 2 Hydrogen atoms 1 oxygen atom. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994.
 Source: chegg.com
Source: chegg.com
225 x 1025 atoms of lead 1 mole 2072g 774419g 7740g 602 x 1023 atoms 1 mole 2. One mole of water contains Avogadros number of molecules ie 6023 1023 water molecules. 10 g gold 1 mole 602 x 1023 atoms 306 x 1022 atoms 1970g 1 mole PRACTICE PROBLEMS. In one mole of water there will exist approximately 6021023 water molecules. So 1 mole H2O 120441024 hydrogen atoms.
 Source: socratic.org
Source: socratic.org
Therefore in order to have 6 water molecules we would need 12 hydrogens and 6 oxygens. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. So there will be a total of 60210232121024 hydrogen atoms. In one mole of water there will exist approximately 602 1023 water molecules. 10 g gold 1 mole 602 x 1023 atoms 306 x 1022 atoms 1970g 1 mole PRACTICE PROBLEMS.
 Source: slideplayer.com
Source: slideplayer.com
If you have a compound like H 2 O then. This means that there are 3 atoms in each molecule. Therefore there will be 6022 1023 oxygen atoms in 1 mole or 18 g of water. One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 10 23 atoms of oxygen. So 1 mole H2O 120441024 hydrogen atoms.
 Source: youtube.com
Source: youtube.com
Now each molecule of water has 2 hydrogen atoms and 1 oxygen atom. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. So 1 mole H2O 120441024 hydrogen atoms. Likewise in order to get 6022 x 1023 molecules of H2O a mole of water we need 12044 x 1023 which is 6022 x 1023 x 2 atoms of hydrogen and 6022 x 1023 atoms of oxygen. Likewise how many oxygen atoms are there in 18 gram of water.

The mass of hydrogen is 1008 gmol and the mass of oxygen is 1600 gmol so the mass of a mole of water can be calculated as follows. How many grams H2O in 1 mol. Therefore there will be 60221023 oxygen atoms in 1 mole or 18 g of water. One mole of water contains 602 x 10 23 MOLECULES of water. You can view more details on each measurement unit.
 Source: slideplayer.com
Source: slideplayer.com
Determine the molar mass of water. 1 water molecule 2 Hydrogen atoms 1 oxygen atom. Since water has a chemical formula of H2O there will be 2 moles of hydrogen in every mole of water. Therefore there will be 6022 1023 oxygen atoms in 1 mole or 18 g of water. But each molecule of water contains 2 H and 1 O atom 3 atoms so there are approximately 18 x 10 24 atoms in a mole of water.
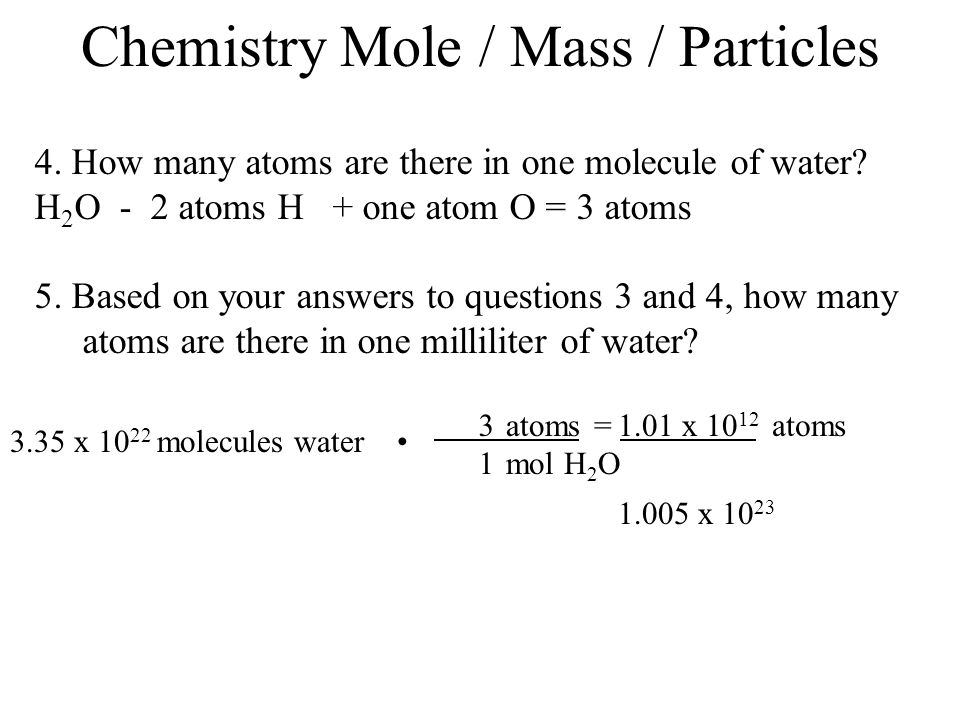 Source: slideplayer.com
Source: slideplayer.com
Mass of 1 Mole of Water Water H2O is made from 2 atoms of hydrogen and 1 atom of oxygen. If you have a compound like H 2 O then. And since the chemical formula for water is H 2O 1 mole of water corresponds to 1 mole of oxygen atoms. 625g of copper 1 mol 977 mol Cu 64 g Cu 2. In this manner how many atoms are in 2 moles of h2o.
 Source: clutchprep.com
Source: clutchprep.com
Since water is H2O one molecule has 3 atoms 2 of hydrogen1 of oxygenSo total atoms 360231023 180691023 atoms. How many atoms are in 100g of gold. Since water has a chemical formula of H2O there will be 2 moles of hydrogen in every mole of water. 1 grams H2O is equal to 0055508435061792 mole. The answer is 1801528.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many atoms are in 1 mole of h2o by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






