Your How many atoms are in 1 mole of h20 images are ready. How many atoms are in 1 mole of h20 are a topic that is being searched for and liked by netizens today. You can Get the How many atoms are in 1 mole of h20 files here. Download all royalty-free photos.
If you’re searching for how many atoms are in 1 mole of h20 images information related to the how many atoms are in 1 mole of h20 topic, you have come to the right blog. Our site always gives you hints for seeking the maximum quality video and image content, please kindly surf and locate more informative video content and images that fit your interests.
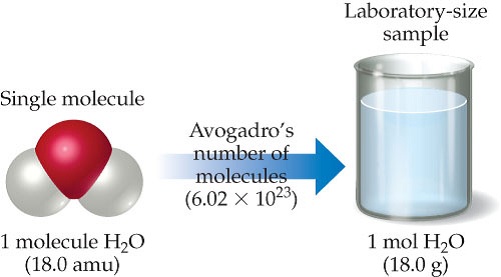
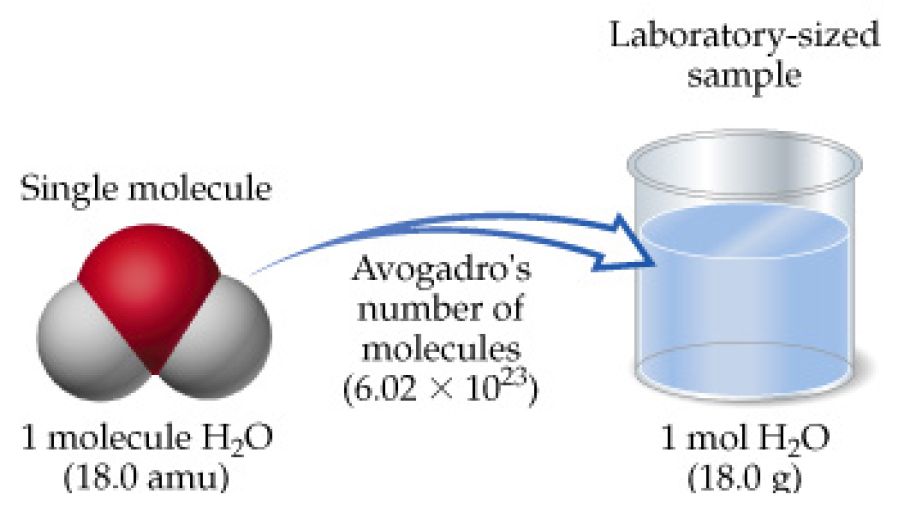
How Many Atoms Are In 1 Mole Of H20. Type in your own numbers in the form to convert the units. What does the 2 represent in h20. And since the chemical formula for water is H 2O 1 mole of water corresponds to 1 mole of oxygen atoms. So 4 moles of H20 would be 24088567161024 water mollecules.
 Solved How Many H2o Molecules Are In A 9 00 G Sample Of Chegg Com From chegg.com
Solved How Many H2o Molecules Are In A 9 00 G Sample Of Chegg Com From chegg.com
How many atoms of oxygen are in one mole of h20. O contains 3 moles of atoms 2 moles of H atoms and 1 mole of O atoms. Here comes the key part. Created by Sal Khan. 1 water molecule 2 Hydrogen atoms 1 oxygen atom. Because the molecular mass of Hydrogen is 1grammole there is 1 mole of hydrogen in 1 gram of hydrogen atoms.
No number means one atom.
Show activity on this post. One mole of a substance is equal to 6022 10²³ units of that substance such as atoms molecules or ions. Therefore there will be 6022 1023 oxygen atoms in 1 mole or 18 g of water. CO2 has 3 atoms 2N20 has 3 atoms and there are already 2 moles so that would be 6 moles of atoms 2NaCl has 2 atoms but again that 2 indicates that youd have 4 moles of atoms and H2SO4 has 7 atoms. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal. How many molecules of water are represented by the formula h2o.
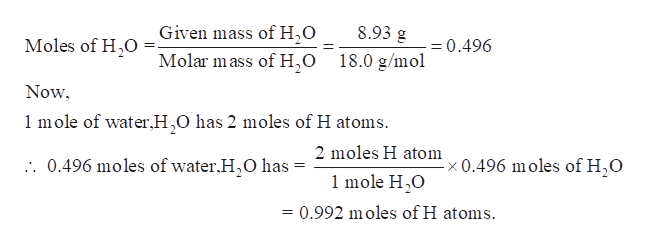 Source: bartleby.com
Source: bartleby.com
The number of atoms or molecules in a mole defined as Avogadros number number of atoms or molecules in one mole of any substance. The concept of the mole can be used to convert between mass and number of particles. And since the chemical formula for water is H 2O 1 mole of water corresponds to 1 mole of oxygen atoms. Type in your own numbers in the form to convert the units. Use this page to learn how to convert between grams H20 and mole.
 Source: lisbdnet.com
Source: lisbdnet.com
The chemical formula for water is H20 which means that every molecule of water has 2 atoms of hydrogen H and one atom of oxygen O. For every mole there are 6021023 atoms so in 1 gram of hydrogen there are 6021023 hydrogen atoms in scientific notation this is equal to 602000000000000000000000 hydrogen atoms. How many atoms of oxygen are in one mole of h20. The number 6022 10²³ is known as Avogadros number or Avogadros constant. From the Periodic Table of Elements one sees that one mole of hydrogen atoms weighs 1 gram while one mole of oxygen atoms weighs 16 grams.
 Source: slideplayer.com
Source: slideplayer.com
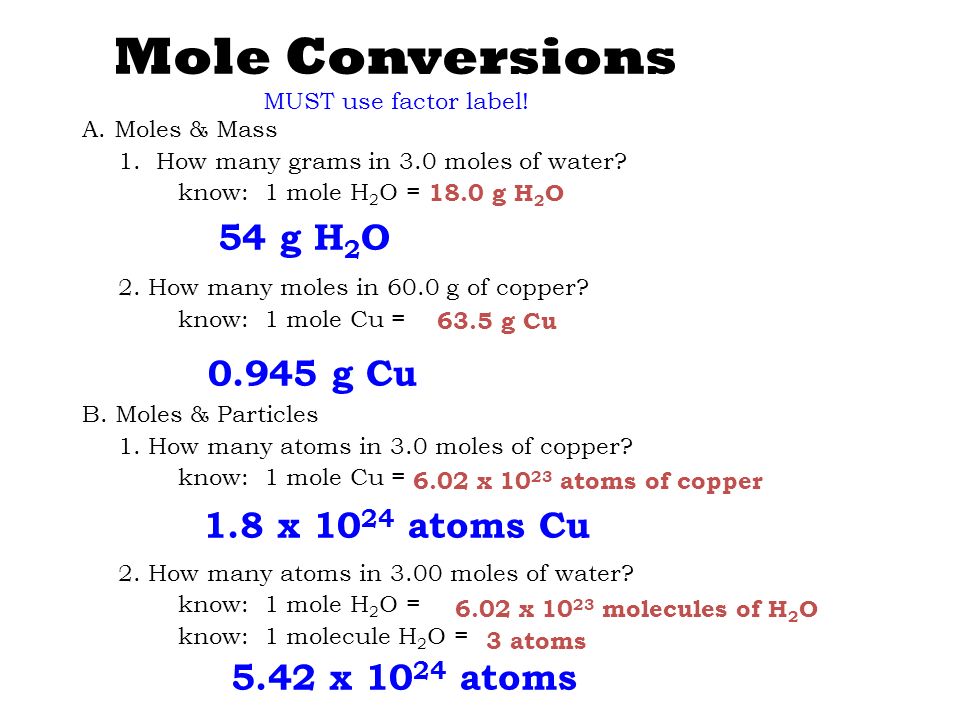
The rule for solving amount of molecules is Nmolecules6x1023x namount of moletherefore there are 6x1023 molecules in 1 mole of anything orin this case of H20. There are 12 of these molecules. How many atoms of oxygen are in one mole of h20. Therefore there will be 6022 1023 oxygen atoms in 1 mole or 18 g of water. The concept of the mole can be used to convert between mass and number of particles.
 Source: slideplayer.com
Source: slideplayer.com
1 mole 60221023 6022 10 23 atoms molecules protons etc. Use the molar masses of each atom together with the number of atoms in the formula and add together. From the Periodic Table of Elements one sees that one mole of hydrogen atoms weighs 1 gram while one mole of oxygen atoms weighs 16 grams. Therefore there will be 60221023 oxygen atoms in 1 mole or 18 g of water. From the Periodic Table of Elements one sees that one mole of hydrogen atoms weighs 1 gram while one mole of oxygen atoms weighs 16 grams.
 Source: chegg.com
Source: chegg.com
Read the material at the link below and work the problems at the end. Here comes the key part. A mole is an important unit because on the periodic table a mole of a substance is equal to its atomic mass in grams. So 1 mole H2O 120441024 hydrogen atoms. From the Periodic Table of Elements one sees that one mole of hydrogen atoms weighs 1 gram while one mole of oxygen atoms weighs 16 grams.
 Source: slideplayer.com
Source: slideplayer.com
Use the molar masses of each atom together with the number of atoms in the formula and add together. There are 12 of these molecules. O contains 3 moles of atoms 2 moles of H atoms and 1 mole of O atoms. This means that 602210 23 carbon atoms or molecules weights 1201 grams. How many atoms of oxygen are in one mole of h20.
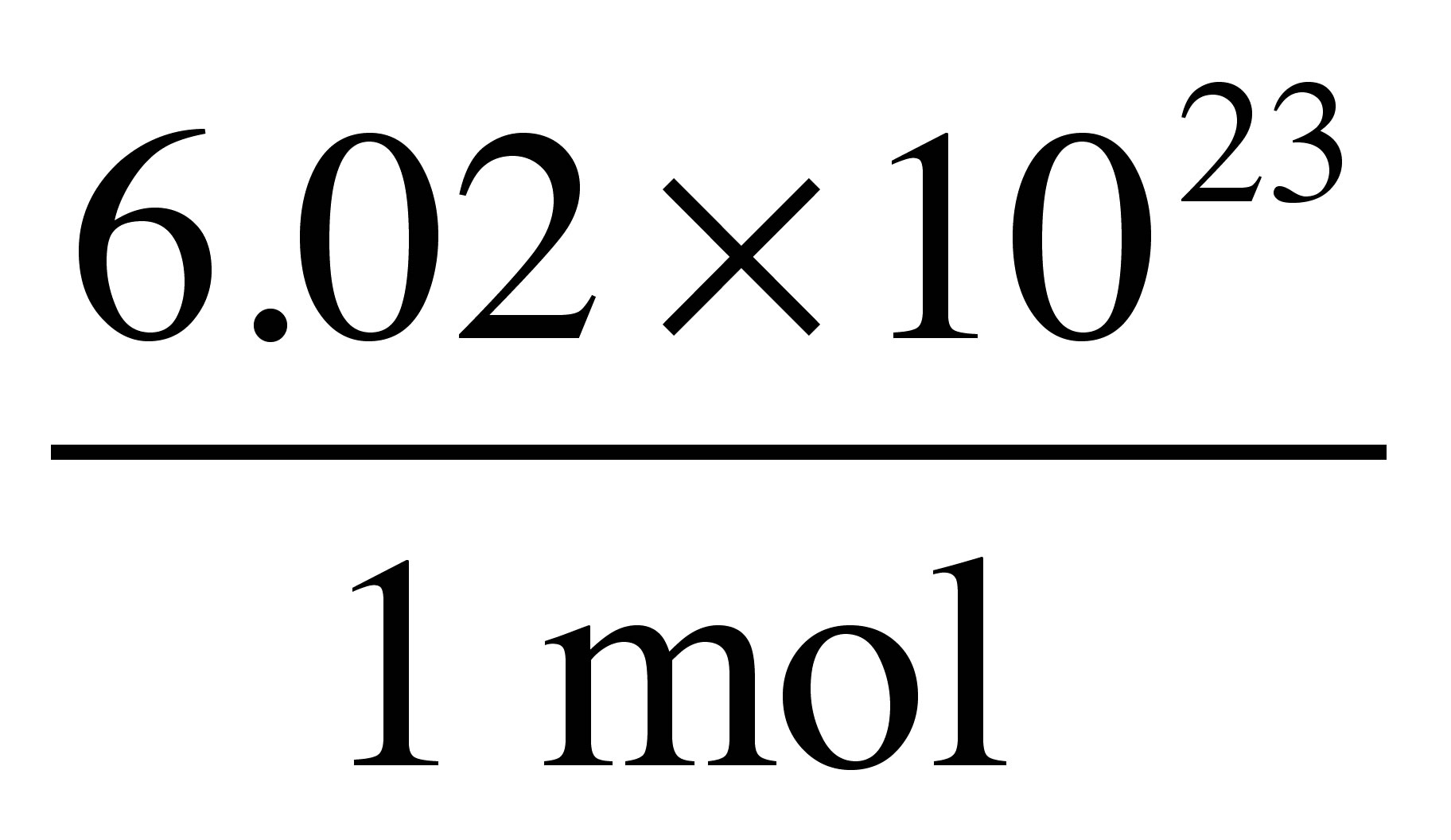 Source: socratic.org
Source: socratic.org
Created by Sal Khan. Note that rounding errors may occur so always check the results. 36021023 1 mole of H 2. The rule for solving amount of molecules is Nmolecules6x1023x namount of moletherefore there are 6x1023 molecules in 1 mole of anything orin this case of H20. How many atoms are in H20.
 Source: slideplayer.com
Source: slideplayer.com
A mole is an important unit because on the periodic table a mole of a substance is equal to its atomic mass in grams. From the Periodic Table of Elements one sees that one mole of hydrogen atoms weighs 1 gram while one mole of oxygen atoms weighs 16 grams. Type in your own numbers in the form to convert the units. So in one molecule there is a total of 3 atoms. Type in your own numbers in the form to convert the units.
 Source: chegg.com
Source: chegg.com
For every mole there are 6021023 atoms so in 1 gram of hydrogen there are 6021023 hydrogen atoms in scientific notation this is equal to 602000000000000000000000 hydrogen atoms. No number means one atom. The chemical formula for water is H20 which means that every molecule of water has 2 atoms of hydrogen H and one atom of oxygen O. What does the 2 represent in h20. 3 moles of atoms corresponds to 36021023 atoms.
 Source: clutchprep.com
Source: clutchprep.com
The number of atoms or molecules in a mole defined as Avogadros number number of atoms or molecules in one mole of any substance. Thus 1 mol of calcium nitrate contains 1 mol of Ca atoms 2 mol of N atoms and 6 mol of O atoms. If you have 6 pairs of shoes you have 12 shoes. 1 grams H20 is equal to 004960612734885 mole. But the number in the case of a mole is 6022141791023.
 Source: slideplayer.com
Source: slideplayer.com
Thus 1 mol of calcium nitrate contains 1 mol of Ca atoms 2 mol of N atoms and 6 mol of O atoms. Thus 1 mol of calcium nitrate contains 1 mol of Ca atoms 2 mol of N atoms and 6 mol of O atoms. How many atoms are in H20. A mole is an important unit because on the periodic table a mole of a substance is equal to its atomic mass in grams. Note that rounding errors may occur so always check the results.
 Source: socratic.org
Source: socratic.org
To convert from moles to atoms multiply the molar amount by Avogadros number. 1 water molecule 2 Hydrogen atoms 1 oxygen atom. O contains 3 moles of atoms 2 moles of H atoms and 1 mole of O atoms. 1 mole 60221023 atoms. Type in your own numbers in the form to convert the units.

So in one molecule there is a total of 3 atoms. Note that rounding errors may occur so always check the results. Plus if there are more water molecules. If you have 6 pairs of shoes you have 12 shoes. There are two things you need to know.

Read the material at the link below and work the problems at the end. In one molecule of water there are 2 atoms of hydrogen for every atom of oxygen. The smaller number is the number of atoms of the element to the left of it. Use this page to learn how to convert between grams H20 and mole. 1 mole is equal to 1 moles H20 or 201588 grams.
 Source: chegg.com
Source: chegg.com
Type in your own numbers in the form to convert the units. Use this page to learn how to convert between moles H20 and gram. This means that 602210 23 carbon atoms or molecules weights 1201 grams. The smaller number is the number of atoms of the element to the left of it. And since the chemical formula for water is H 2O 1 mole of water corresponds to 1 mole of oxygen atoms.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many atoms are in 1 mole of h20 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






