Your How many atoms are in 1 mole of co2 images are available in this site. How many atoms are in 1 mole of co2 are a topic that is being searched for and liked by netizens today. You can Find and Download the How many atoms are in 1 mole of co2 files here. Find and Download all royalty-free photos and vectors.
If you’re searching for how many atoms are in 1 mole of co2 images information related to the how many atoms are in 1 mole of co2 topic, you have visit the right blog. Our site frequently gives you suggestions for seeking the highest quality video and picture content, please kindly surf and locate more informative video articles and images that fit your interests.
How Many Atoms Are In 1 Mole Of Co2. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. 01 mole incorporates 2. Now in the question the given compound CO2 there are two oxygen atoms and one carbon atom. 1 mole carbon dioxide contains 602 x 1023 molecules.

How many moles of carbon atoms are there in 0500 mole of C2H6. A mole is a convenient counting unit whenever one is dealing with numbers of atoms or molecules. 1 mole carbon dioxide contains 602 x 1023 molecules. 1 mole of CO2 1 mole of C 602 10 23 atoms of carbon. Chemistry University of Wisconsin-Madison 1982 Answered 2 years ago Author has 33K answers and 59M answer views. Subsequently How many atoms are there in one mole of carbon dioxide.
Therefore there are 6022 x 1023 atoms of carbon and 12044 x 1023 atoms of oxygen in that 1 mole of CO2.
1200 g C-12 1 mol C-12 atoms 6022 x 1023 atomsThank you. Lets consider the carbon dioxide molecule. Now in the question the given compound CO2 there are two oxygen atoms and one carbon atom. How many atoms are there in one mole of carbon dioxide. 1 mol C 1201 g C or 1201 g C 1 mol C Changing between Mass and Variety of Moles Changing between Mass and Variety of Atoms Calculate the moles of carbon in 00265 g of pencil lead. 1 mole carbon dioxide contains 602 x 1023 molecules.

Now in the question the given compound CO2 there are two oxygen atoms and one carbon atom. Now in the question the given compound CO2 there are two oxygen atoms and one carbon atom. A mole is a convenient counting unit whenever one is dealing with numbers of atoms or molecules. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. CH4g 2O2g CO2g 2H2Og A single replacement.
 Source: chegg.com
Source: chegg.com
1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. 1 mole is equal to 1 moles Oxygen or 159994 grams. 1200 g C-12 1 mol C-12 atoms 6022 x 1023 atomsThank you. How many moles of carbon atoms are there in 0500 mole of C2H6. Related Links How Many Different Words Can Be Formed By Jumbling The Letters In The Word Mississippi.

How many moles of carbon atoms are there in 0500 mole of C2H6. Avogadros number shows us that there are 6022 x 1023 molecules of CO2 in 1 mole of the gas. Now in the question the given compound CO2 there are two oxygen atoms and one carbon atom. 1 mol C 1201 g C or 1201 g C 1 mol C Changing between Mass and Variety of Moles Changing between Mass and Variety of Atoms Calculate the moles of carbon in 00265 g of pencil lead. Therefore one mole of CO2 contains one mole of carbon and two moles of oxygen.
 Source: slideplayer.com
Source: slideplayer.com
Considering this how many atoms are in 750 moles of zinc. Subsequently How many atoms are there in one mole of carbon dioxide. Lets consider the carbon dioxide molecule. Chemistry University of Wisconsin-Madison 1982 Answered 2 years ago Author has 33K answers and 59M answer views. 1 mol C 1201 g C or 1201 g C 1 mol C Changing between Mass and Variety of Moles Changing between Mass and Variety of Atoms Calculate the moles of carbon in 00265 g of pencil lead.
 Source: youtube.com
Source: youtube.com
There are three atoms in the compound CO2 one carbon and two oxygen atoms. Chemistry University of Wisconsin-Madison 1982 Answered 2 years ago Author has 33K answers and 59M answer views. How many moles of carbon atoms are there in 0500 mole of C2H6. 1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. It is equal to Avogadros number NA namely 6022 x10 23If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams.

In 1 mole there are roughly 6023 1023 elements here molecules. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. We know it has the formula CO2 and this tells us that. Now in the question the given compound CO2 there are two oxygen atoms and one carbon atom.
 Source: toppr.com
Source: toppr.com
But each molecule of water contains 2 H and 1 O atom 3 atoms so there are approximately 18 x 10 24 atoms in a mole of water. Was this reply useful. A 0500 moles B 100 moles C 300 moles D 602 1023 moles. Considering this how many atoms are in 750 moles of zinc. We know it has the formula CO2 and this tells us that.

Considering this how many atoms are in 750 moles of zinc. There are three atoms in the compound CO2 one carbon and two oxygen atoms. 6022 1023 2mols 12044 1024. We know it has the formula CO2 and this tells us that. We are able to reply these questions as follows.
 Source: slideplayer.com
Source: slideplayer.com
1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. The number of atoms in any mole of substance is always 6022 1023 however the mass of one mole will differ between compounds. 1 mole carbon dioxide contains 602 x 1023 molecules. A mole is a convenient counting unit whenever one is dealing with numbers of atoms or molecules. 6022 1023 2mols 12044 1024.
 Source: slideplayer.com
Source: slideplayer.com
Avogadros quantity exhibits us that there are 6022 x 1023 molecules of CO2 in 1 mole of the fuel. Lets consider the carbon dioxide molecule. 1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. We know it has the formula CO2 and this tells us that. One mole of any element or 1 g atom 602 10 23 atoms.
 Source: sites.google.com
Source: sites.google.com
The value of the mole in precisely 12 grammes of pure carbon-12 is equal to the number of atoms. The reaction of methane with oxygen to produce carbon dioxide and water is an example of which class of reaction. The SI base unit for amount of substance is the mole. In 1 mole there are roughly 6023 1023 elements here molecules. 10 24 oxygen atoms.

Now in the question the given compound CO2 there are two oxygen atoms and one carbon atom. Therefore there are 6022 x 1023 atoms of carbon and 12044 x. 1 mol C 1201 g C or 1201 g C 1 mol C Changing between Mass and Variety of Moles Changing between Mass and Variety of Atoms Calculate the moles of carbon in 00265 g of pencil lead. Therefore there are 6022 x 1023 atoms of carbon and 12044 x 1023 atoms of oxygen in that 1 mole of CO2. 1 mole carbon dioxide contains 602 x 1023 molecules.
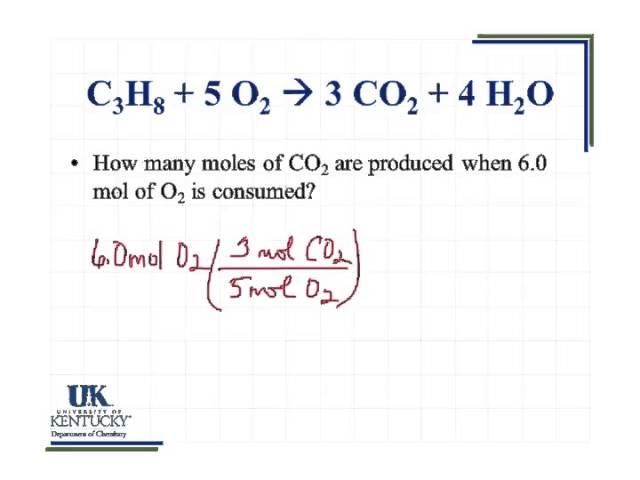 Source: youtube.com
Source: youtube.com
The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. An element is a substance made of a single type of atom. Lets consider the carbon dioxide molecule. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. We know it has the formula CO2 and this tells us that.

Therefore there are 6022 x 1023 atoms of carbon and 12044 x 1023 atoms of oxygen in that 1 mole of CO2. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. 10 24 oxygen atoms. 01 mole incorporates 2. In CO2 there are 3 atoms per molecule.

Therefore there are 6022 x 1023 atoms of carbon and 12044 x. Chemistry University of Wisconsin-Madison 1982 Answered 2 years ago Author has 33K answers and 59M answer views. Avogadros number shows us that there are 6022 x 1023 molecules of CO2 in 1 mole of the gas. There are three atoms in the compound CO2 one carbon and two oxygen atoms. Was this reply useful.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many atoms are in 1 mole of co2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.