Your How do you turn grams to moles images are available in this site. How do you turn grams to moles are a topic that is being searched for and liked by netizens now. You can Get the How do you turn grams to moles files here. Download all royalty-free photos and vectors.
If you’re searching for how do you turn grams to moles pictures information linked to the how do you turn grams to moles topic, you have come to the right blog. Our website frequently gives you hints for viewing the maximum quality video and picture content, please kindly hunt and locate more enlightening video articles and images that match your interests.
How Do You Turn Grams To Moles. Multiply the number of moles by the molar mass to obtain the final answer in grams. Divide the number of grams of the compound by its molecular mass. How many grams are in 379 moles of calcium bromide CaBr 2. Using a calculator divide the number of grams by the molar mass.
 How To Convert Grams To Moles 8 Steps With Pictures Wikihow From wikihow.com
How To Convert Grams To Moles 8 Steps With Pictures Wikihow From wikihow.com
1 grams H2 is equal to 04960612734885 mole. To do so use the following figure. This is the weight in grams of one mole of the element. Using a calculator divide the number of grams by the molar mass. Formula to convert moles to grams. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams.
Example 37 x 1024 atoms1 x 1mole Na6022 x 1023 atoms Na x 23g Na1mole Na.
The answer is 201588. 1 mole is equal to 1 moles In or 114818 grams. We assume you are converting between grams H2 and mole. The molar mass of H 2 O 2 is 340146 gramsmole. Grams to Moles Conversion Formula. Multiply the moles given by the substances molar mass.
 Source: slideplayer.com
Source: slideplayer.com
Convert 02 moles of Sodium chloride. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. 1 grams H2 is equal to 04960612734885 mole. See an example of converting grams to moles. Find the molecular mass of the compound.
 Source: study.com
Source: study.com
Convert 02 moles of Sodium chloride. Pick one of the possible groups of compounds you can choose one from Common Gases and Liquids Salts Acids Alkali. You can also enter any custom value for molar mass. This is the weight in grams of one mole of the element. Convert 02 moles of Sodium chloride.
 Source: study.com
Source: study.com
Multiply both the values. Using a calculator divide the number of grams by the molar mass. Find the molecular mass of the compound. Formula to convert moles to grams. 02 x 5844 11688 grams.
 Source: wikihow.com
Source: wikihow.com
1 mole is equal to 1 moles In or 114818 grams. This is the weight in grams of one mole of the element. Convert 02 moles of Sodium chloride. We can now convert 100g of NaOH to moles. Divide the number of grams of the compound NaOH by the molecular weight and as a result the grams g unit cancels and all we have left is the unit mol.
 Source: study.com
Source: study.com
Number of grams molar mass number of moles. To convert moles into grams determine the number of moles preset and the molar mass of the compound. The tool is really user friendly and pretty straightforward to use. Molecular weight of In or grams. 1 grams H2 is equal to 04960612734885 mole.
 Source: youtube.com
Source: youtube.com
Converting grams to moles involves 2 steps. Grams Moles x Molar Mass. Example 1 Calculate the mass in grams of 36 mol of H2SO4. The molar mass of H 2 O 2 is 340146 gramsmole. Divide the number of grams of the substance by the molecular mass.
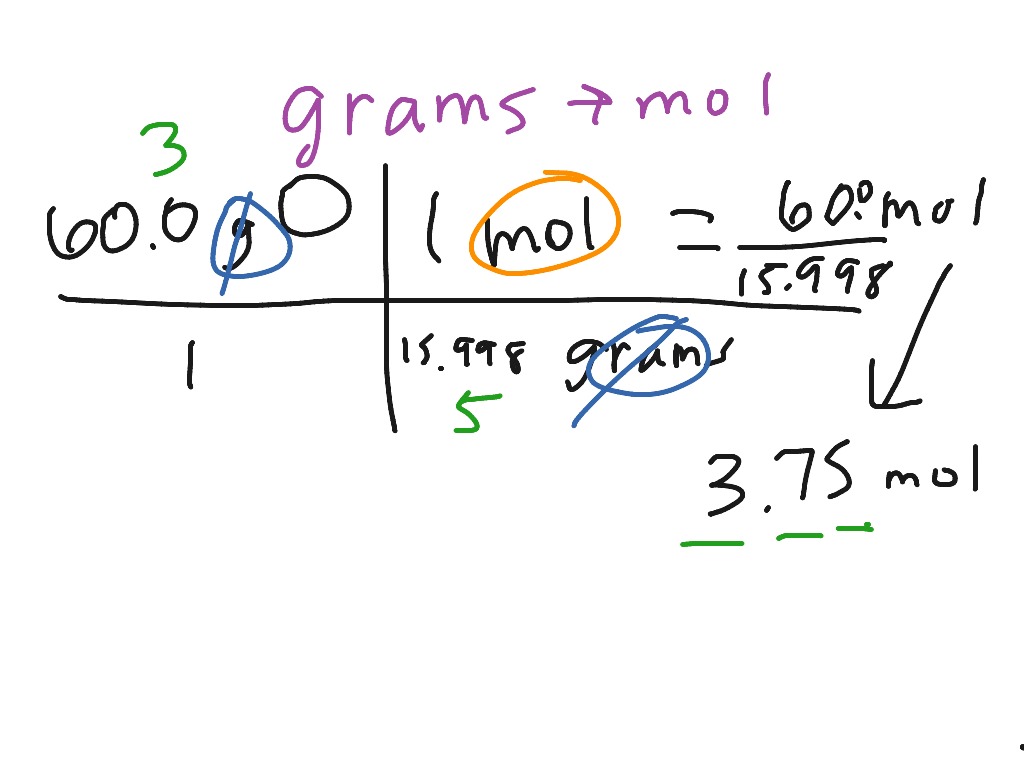 Source: showme.com
Source: showme.com
Molecular weight of In or grams. Figure 112 By using this map as a guide you can easily learn to convert between grams molecules and moles of. This is the weight in grams of one mole of the element. The answer is 201588. Using a calculator divide the number of grams by the molar mass.
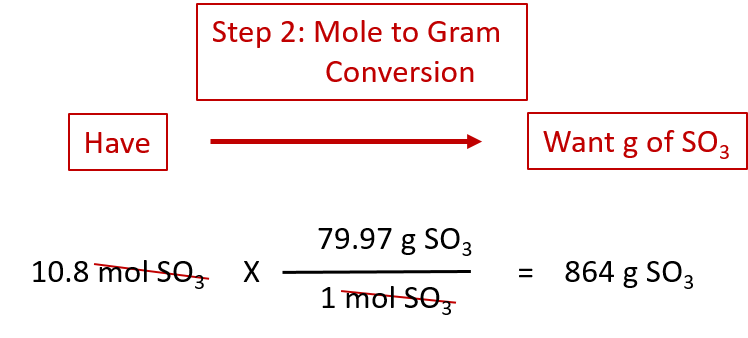 Source: wou.edu
Source: wou.edu
Figure 112 By using this map as a guide you can easily learn to convert between grams molecules and moles of. How do you turn grams into moles. 0700 mole x 340146 gramsmole 238 grams. Divide the number of grams of the compound NaOH by the molecular weight and as a result the grams g unit cancels and all we have left is the unit mol. In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight of the compound in question.
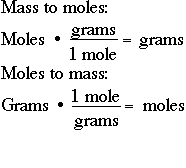 Source: socratic.org
Source: socratic.org
How many grams are in 0572 moles of glucose C 6 H 12 O 6. Using a calculator divide the number of grams by the molar mass. The SI base unit for amount of substance is the mole. Many chemical equations require that the amount of substance be in the unit of moles. Converting grams to moles involves 2 steps.
 Source: youtube.com
Source: youtube.com
Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. Multiply the number of moles by the molar mass to obtain the final answer in grams. The answer is 201588. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. How many grams are in 379 moles of calcium bromide CaBr 2.
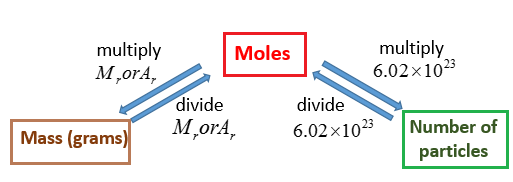 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Convert 02 moles of Sodium chloride. Multiply both the values. The tool is really user friendly and pretty straightforward to use. For example imagine you have 2 g of water or H 2 O and you want to convert it. The answer will be the number of moles of the compound.
 Source: youtube.com
Source: youtube.com
Multiply the moles given by the substances molar mass. How many grams are in 0572 moles of glucose C 6 H 12 O 6. In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight of the compound in question. Many chemical equations require that the amount of substance be in the unit of moles. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula.
 Source: youtube.com
Source: youtube.com
Convert 02 moles of Sodium chloride. To convert moles into grams determine the number of moles preset and the molar mass of the compound. The tool is really user friendly and pretty straightforward to use. In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight of the compound in question. Divide the number of grams of the substance by the molecular mass.
 Source: youtube.com
Source: youtube.com
How many grams are in 0572 moles of glucose C 6 H 12 O 6. We can now convert 100g of NaOH to moles. 0700 mole x 340146 gramsmole 238 grams. The answer will be the number of moles of the compound. To convert moles into grams determine the number of moles preset and the molar mass of the compound.
 Source: chem.fsu.edu
Source: chem.fsu.edu
The number of moles present in a compound is often given to the student in the problem. How do you turn grams into moles. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. This leads to a very common conversion in which the grams of the substance be converted into moles. Example 1 Calculate the mass in grams of 36 mol of H2SO4.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how do you turn grams to moles by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






