Your How do you go from moles to atoms images are available in this site. How do you go from moles to atoms are a topic that is being searched for and liked by netizens today. You can Get the How do you go from moles to atoms files here. Get all royalty-free photos and vectors.
If you’re looking for how do you go from moles to atoms pictures information connected with to the how do you go from moles to atoms keyword, you have visit the ideal blog. Our site frequently provides you with suggestions for viewing the maximum quality video and image content, please kindly surf and find more enlightening video articles and graphics that match your interests.
How Do You Go From Moles To Atoms. One mole of water contains 602 x 10 23 MOLECULES of water. The mole is represented by Avogadros number which is 60221023 atoms or molecules per mol. Molar Mass Get the Gizmo ready. You divide and find that 1 gram of fluorine is equal to 00525350025878 moles.
 As My Father Always Said When In Doubt Convert To Moles How To Convert Between Grams Moles And At Chemistry Lessons Chemistry Classroom Teaching Chemistry From br.pinterest.com
As My Father Always Said When In Doubt Convert To Moles How To Convert Between Grams Moles And At Chemistry Lessons Chemistry Classroom Teaching Chemistry From br.pinterest.com
3 arrows 3 conversion. 2 mole to atom 12044283E24 atom. People found this article helpful. Note that rounding errors may occur so always check the results. 1 mole to atom 60221415E23 atom. One mole of water contains 602 x 10 23 MOLECULES of water.
100 x 10 22 atoms Activity A.
How do you convert MOL to atoms. Lets say you have 25 moles of Au Gold and youre really curious as to how many atoms of gold are in. B moles x 60221023 atoms 1 mole C atoms. Since atoms are so tiny chemists have devised a unit known as the moleA mole represents a macroscopic quantity of matter that can be used in the laboratory. One mole of any element. Type in your own numbers in the form to convert the units.
 Source: in.pinterest.com
Source: in.pinterest.com
This chemistry video tutorial explains the conversion process of grams to atoms which is useful in solving common stoichiometry practice problems. Avogadros number is a very important relationship to remember. The equation to convert moles to atoms is as follows. Quick conversion chart of molecules to atoms. You divide and find that 1 gram of fluorine is equal to 00525350025878 moles.
 Source: pinterest.com
Source: pinterest.com
The example will use both the bridge concept and conversion grids. As a simple example a molecule of water H2O is made out of one hydrogen atom and two oxygen atoms. When going from molecules to moles you divide by 602 x 10 23. 3 arrows 3 conversion. One mole of water contains 602 x 10 23 MOLECULES of water.
 Source: pinterest.com
Source: pinterest.com
This chemistry video tutorial explains the conversion process of grams to atoms which is useful in solving common stoichiometry practice problems. We pass through 3 arrows when we go from Grams Moles Molecules Atoms. Moles to atoms you multiply the number of moles by avogadros number ex. 2 mole to atom 12044283E24 atom. 6022 x 10 23 atoms or molecules 1 mole remember Avogadro.
 Source: nl.pinterest.com
Source: nl.pinterest.com
Then you multiply that by your 878 grams. We pass through 3 arrows when we go from Grams Moles Molecules Atoms. Look at the conversion map. To convert from moles to atoms multiply the molar amount by Avogadros number. 1 Mole 60221415E23 Atom.
 Source: pinterest.com
Source: pinterest.com
But each molecule of water contains 2 H and 1 O atom 3 atoms so there are approximately 18 x 10 24 atoms in a mole of water. 1 mole to atom 60221415E23 atom. 5g carbon 0417 mols of carbon 12 gmol How many moles of oxygen are in 12 g of CO 2. Then you multiply that by your 878 grams. Look at the conversion map.
 Source: pinterest.com
Source: pinterest.com
Calculating And Converting Moles and Atoms. Type in your own numbers in the form to convert the units. 100 x 10 22 atoms Activity A. Determine the number of grams from moles of E. 1 mole is equal to 60221415E23 molecules or 60221415E23 atoms.
 Source: pinterest.com
Source: pinterest.com
To convert between moles and molecules you need to remember that one mole of any substance contains 602 x 10 23 particles eg atoms or molecules. To convert from moles to atoms multiply the molar amount by Avogadros number. To get moles from atoms divide number of atoms by 6022 x 1023. Your job is to create fractions that will take you the way you need to go. Note that rounding errors may occur so always check the results.
 Source: pinterest.com
Source: pinterest.com
To get atoms from moles multiply number of moles by 6022 x 1023. After you get that answer you can use Avagadros number 6022X1023 to find the atoms. How do you convert MOL to atoms. The equation to convert moles to atoms is as follows. How Do You Convert Moles To Atoms.
 Source: hu.pinterest.com
Source: hu.pinterest.com
12 g CH 4. One mole of water contains 602 x 10 23 MOLECULES of water. Atoms or moles The SI base unit for amount of substance is the mole. Avogadros number is a very important relationship to remember. Type in your own numbers in the form to convert the units.
 Source: pinterest.com
Source: pinterest.com
Add up the atomic mass of all the elements. Atoms or moles The SI base unit for amount of substance is the mole. Determine the number of grams from moles of E. 1 atoms is equal to 127E-24 mole. Use this page to learn how to convert between molecules and atoms.
 Source: pinterest.com
Source: pinterest.com
We pass through 3 arrows when we go from Grams Moles Molecules Atoms. Type in your own numbers in the form to convert the units. You divide and find that 1 gram of fluorine is equal to 00525350025878 moles. Moles to Atoms Write this. Your job is to create fractions that will take you the way you need to go.
 Source: pinterest.com
Source: pinterest.com
To convert moles to atomsatoms Cs 0404 mol Cs602 x 1023 atoms 243E23 atoms CsmolMultiply by atomsmole. 12g CO 2 X 2 atoms. The equation to convert moles to atoms is as follows. To get atoms from moles multiply number of moles by 6022 x 1023. The example will use both the bridge concept and conversion grids.
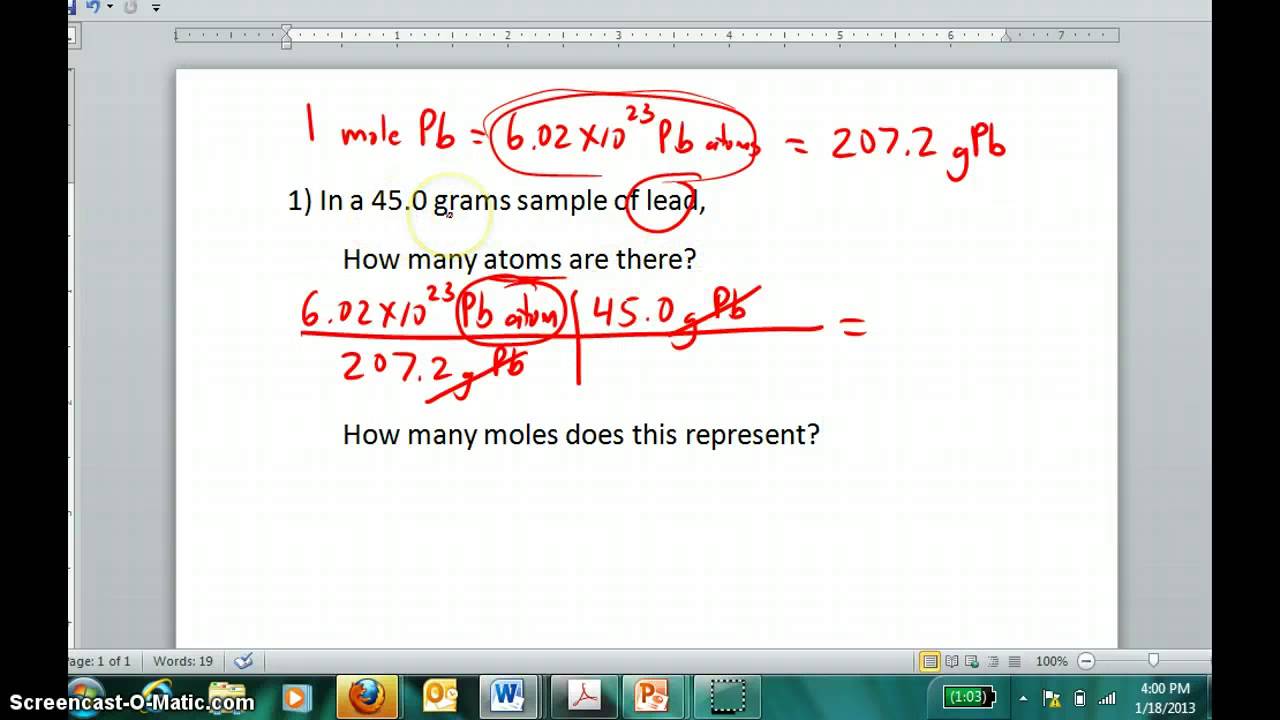 Source: pinterest.com
Source: pinterest.com
One mole of any element. Determine the number of grams from moles of E. After you get that answer you can use Avagadros number 6022X1023 to find the atoms. 1 mole to atom 60221415E23 atom. This is due to the fact that the number of atoms in a mole is chosen in such a way for this to happen 602 x 1023.
 Source: pinterest.com
Source: pinterest.com
100 x 10 22 atoms Activity A. This is due to the fact that the number of atoms in a mole is chosen in such a way for this to happen 602 x 1023. First convert atoms of oxygen to molecules of sulfuric acid. Your job is to create fractions that will take you the way you need to go. Then you multiply that by your 878 grams.
 Source: br.pinterest.com
Source: br.pinterest.com
This is due to the fact that the number of atoms in a mole is chosen in such a way for this to happen 602 x 1023. Where Atom Number of atoms. Type in your own numbers in the form to convert the units. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal. This tutorial contains plen.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how do you go from moles to atoms by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





