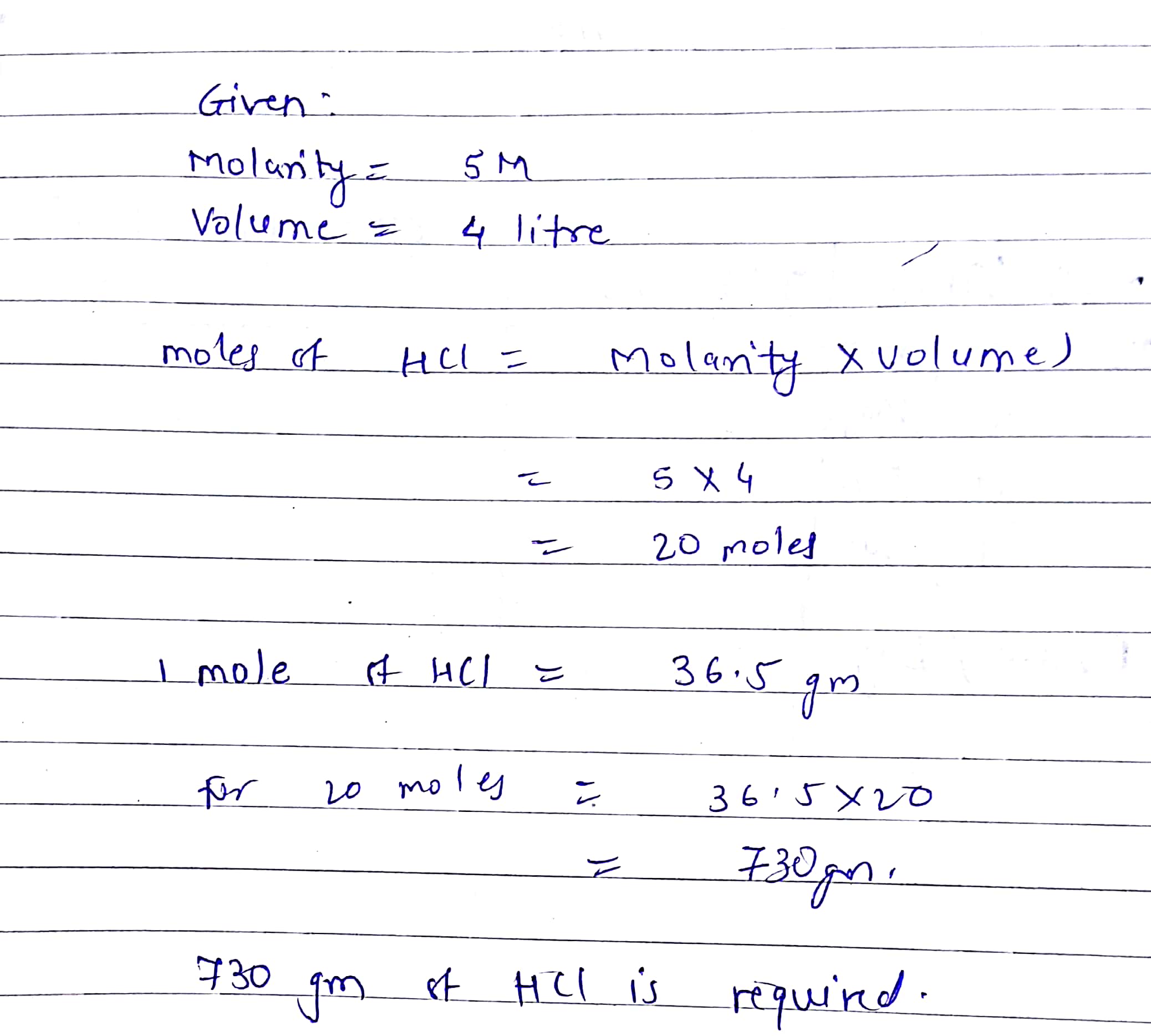Your How do you go from atoms to moles images are ready. How do you go from atoms to moles are a topic that is being searched for and liked by netizens now. You can Download the How do you go from atoms to moles files here. Find and Download all royalty-free vectors.
If you’re searching for how do you go from atoms to moles images information linked to the how do you go from atoms to moles topic, you have come to the right blog. Our website always gives you suggestions for downloading the maximum quality video and image content, please kindly surf and find more enlightening video content and graphics that match your interests.
How Do You Go From Atoms To Moles. 3612 x 1023 atoms Fe. The Avogadros Constant is equal to 6. Note that rounding errors may occur so always check the results. People found this article helpful.
 Chemistry Lessons Chemistry Classroom Teaching Chemistry From pinterest.com
Chemistry Lessons Chemistry Classroom Teaching Chemistry From pinterest.com
To transform from atoms to moles divide the atom quantity by Avogadros quantity or multiply by its reciprocal. Lets plug these numbers into the above equation. First convert atoms of oxygen to molecules of sulfuric acid. That should give you the proper amount of moles. When going from moles to molecules you multiply by 602 x 10 23. Do a quick conversion.
6 days ago 2314 mole Cd x 6022 x 10 atoms Cd 84 x 1023 atoms Cd 1 mole Cd 4 How many moles are in 43 x 1022 molecules of H 3 PO 4.
Instead of multiplying the number of moles by Avogadros number youd divide the number of atoms by Avagadros number. 1 Mole 60221415E23 Atom. To convert from moles to atoms multiply the molar amount by Avogadros number. To get moles from atoms divide number of atoms by 6022 x 1023. How Do You Convert Moles To Atoms. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal.
 Source: pinterest.com
Source: pinterest.com
Your job is to create fractions that will take you the way you need to go. 1 atoms 1660538863127E-24 moles using the online calculator for metric conversions. Where Atom Number of atoms. 6 days ago 2314 mole Cd x 6022 x 10 atoms Cd 84 x 1023 atoms Cd 1 mole Cd 4 How many moles are in 43 x 1022 molecules of H 3 PO 4. Then you multiply that by your 878 grams.
 Source: pinterest.com
Source: pinterest.com
When going from moles to molecules you multiply by 602 x 10 23. 1 atoms 1660538863127E-24 moles using the online calculator for metric conversions. Do a quick conversion. To get atoms from moles multiply number of moles by 6022 x 1023. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal.
 Source: pinterest.com
Source: pinterest.com
To transform from moles to atoms multiply the molar quantity by Avogadros quantity. All that needs to be done is the above calculation but in reverse. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal. We know we have 10 g of HCl and it has a molecular weight of 365 g mol. The Mega Mole Worksheet 1-10 Convert to Moles 1204 x 1023 atoms He.
 Source: in.pinterest.com
Source: in.pinterest.com
1 Mole 60221415E23 Atom. Check the chart for more details. Atom Mole 60221415E23. When going from molecules to moles you divide by 602 x 10 23. To convert atoms to moles you need to take the amount of atoms that you have multiply it by 1 mol Avagadros number.
 Source: pinterest.com
Source: pinterest.com
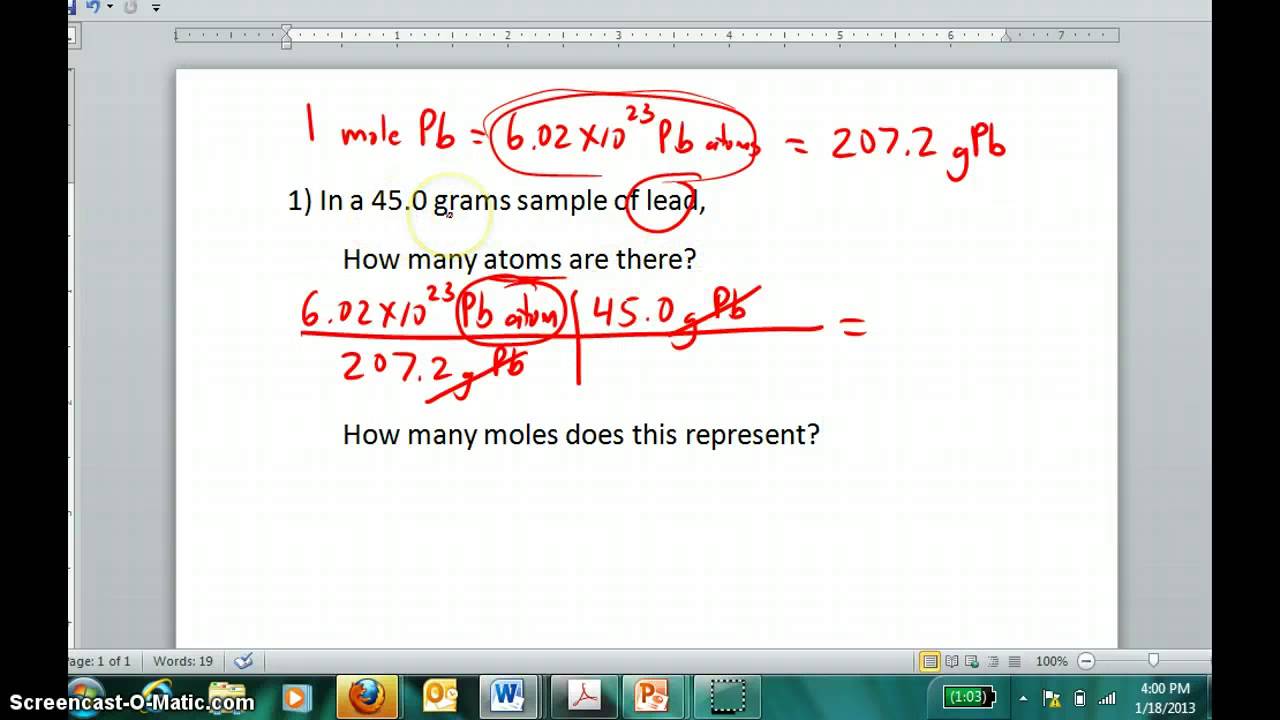
That should give you the proper amount of moles. Example problem converting atoms of an element to moles of that element. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. The atom is the smallest particle of a chemical element that can exist. 1 atoms is equal to 127E-24 mole.
 Source: pinterest.com
Source: pinterest.com
For elemental oxygen the atomic mass is 16 so. Lets plug these numbers into the above equation. Do a quick conversion. 1 atoms 1660538863127E-24 moles using the online calculator for metric conversions. 1 mole 60221023 6022 10 23 atoms molecules protons etc.
 Source: pinterest.com
Source: pinterest.com
301 x 1023 atoms Cu. 301 x 1023 atoms Cu. To convert moles of atoms divide the atom amount by Avogadros number. Your job is to create fractions that will take you the way you need to go. C atoms X 1 mole60221023 atoms B moles.
 Source: pinterest.com
Source: pinterest.com
Where Atom Number of atoms. Quickly convert atoms into moles using this online atoms to moles calculator. 1 Mole 60221415E23 Atom. Avogadros number is a very important relationship to remember. 1 atoms 1660538863127E-24 moles using the online calculator for metric conversions.
 Source: pinterest.com
Source: pinterest.com
The Mega Mole Worksheet 1-10 Convert to Moles 1204 x 1023 atoms He. To convert between moles and molecules you need to remember that one mole of any substance contains 602 x 10 23 particles eg atoms or molecules. Avogadros number is a very important relationship to remember. 3612 x 1023 atoms Fe. This tutorial contains plen.
 Source: br.pinterest.com
Source: br.pinterest.com
Its very easy to convert the number of atoms in a substance to some number of moles as well. Atom Mole 60221415E23. That should give you the proper amount of moles. When going from moles to molecules you multiply by 602 x 10 23. For elemental oxygen the atomic mass is 16 so.
 Source: hu.pinterest.com
Source: hu.pinterest.com
1 mole 60221023 6022 10 23 atoms molecules protons and so on. When going from molecules to moles you divide by 602 x 10 23. Its very easy to convert the number of atoms in a substance to some number of moles as well. 1 mole 60221023 6022 10 23 atoms molecules protons and so on. We know we have 10 g of HCl and it has a molecular weight of 365 g mol.
 Source: pinterest.com
Source: pinterest.com
The atom is the smallest particle of a chemical element that can exist. How do you convert MOL to atoms. To convert from moles to atoms multiply the molar amount by Avogadros number. When going from molecules to moles you divide by 602 x 10 23. Lets plug these numbers into the above equation.
 Source: pinterest.com
Source: pinterest.com
C atoms X 1 mole60221023 atoms B moles. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. The Mega Mole Worksheet 1-10 Convert to Moles 1204 x 1023 atoms He. Avogadros number is a very important relationship to remember.
 Source: pinterest.com
Source: pinterest.com
The Mega Mole Worksheet 1-10 Convert to Moles 1204 x 1023 atoms He. Atoms or moles The SI base unit for amount of substance is the mole. Avogadros number is a very important relationship to remember. Check the chart for more details. Atoms To Moles Calculator.
 Source: pinterest.com
Source: pinterest.com
To get atoms from moles multiply number of moles by 6022 x 1023. To transform from moles to atoms multiply the molar quantity by Avogadros quantity. Where Atom Number of atoms. The atom is the smallest particle of a chemical element that can exist. We know we have 10 g of HCl and it has a molecular weight of 365 g mol.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how do you go from atoms to moles by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.