Your How do you find the number of grams per mole for an element images are available. How do you find the number of grams per mole for an element are a topic that is being searched for and liked by netizens today. You can Get the How do you find the number of grams per mole for an element files here. Get all royalty-free images.
If you’re looking for how do you find the number of grams per mole for an element images information related to the how do you find the number of grams per mole for an element interest, you have come to the right blog. Our website always gives you suggestions for viewing the highest quality video and picture content, please kindly hunt and locate more informative video content and images that match your interests.
How Do You Find The Number Of Grams Per Mole For An Element. 1 day ago One mole consists of Avogadro number of atomsIf you know the quantity of mole it can be. Fifth convert to grams. Therefore the molecular mass of H 2 SO 4 is. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula.
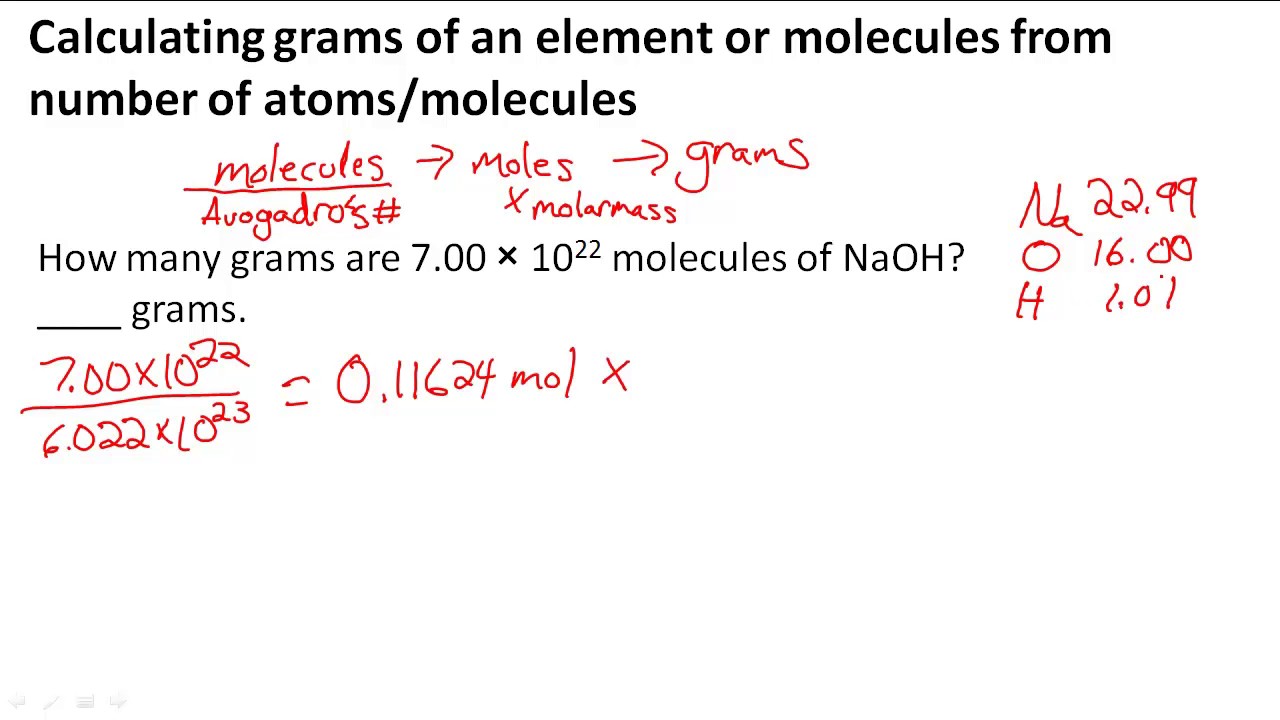 Calculating Grams Of An Element Or Molecules From Number Of Atoms Molecules Youtube From youtube.com
Calculating Grams Of An Element Or Molecules From Number Of Atoms Molecules Youtube From youtube.com
N the number of moles of a compound. Im told these are incorrect answers. The unit is typically gmol. To go from grams to moles divide the grams by the molar mass. So The number of moles of each element. The quotient tells you the number of moles.
Definition of Number of Moles.
If you have a sample that contains only atoms of a particular element weigh the sample in grams and divide by the atomic weight of the element. N the number of moles of a compound. 170 mol 58443 g 1 mol 994 g. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula. It refers to a huge number that we use to measure atoms. Moreover it is equal to the number of atoms in 12 grams of carbon-12 that is.
 Source: pinterest.com
Source: pinterest.com
For example imagine you have 2 g of water or H 2 O and you want to convert it to molesThe molecular mass of H 2 O is 18gmol. Then 1000 g 151001 gmol. More free chemistry help videos. If you have 1000 grams. To go from grams to moles divide the grams by the molar mass.
 Source: youtube.com
Source: youtube.com
The unit is typically gmol. Number of moles Weight of compound in grams molecular weight of compound. Calculate Moles of Product. Multiply the elements atomic mass by the number of atoms of that element in the compound. You now have calculated the number of moles of every compound used in this reaction.
 Source: pinterest.com
Source: pinterest.com
Multiply that by Avogadros number and youll find out how many atoms the sample contains. Look for the atomic masses of hydrogen sulfur and oxygen. This will give you the relative amount that each element contributes to the. N the number of moles of a compound. The formula for moles to grams is given by.
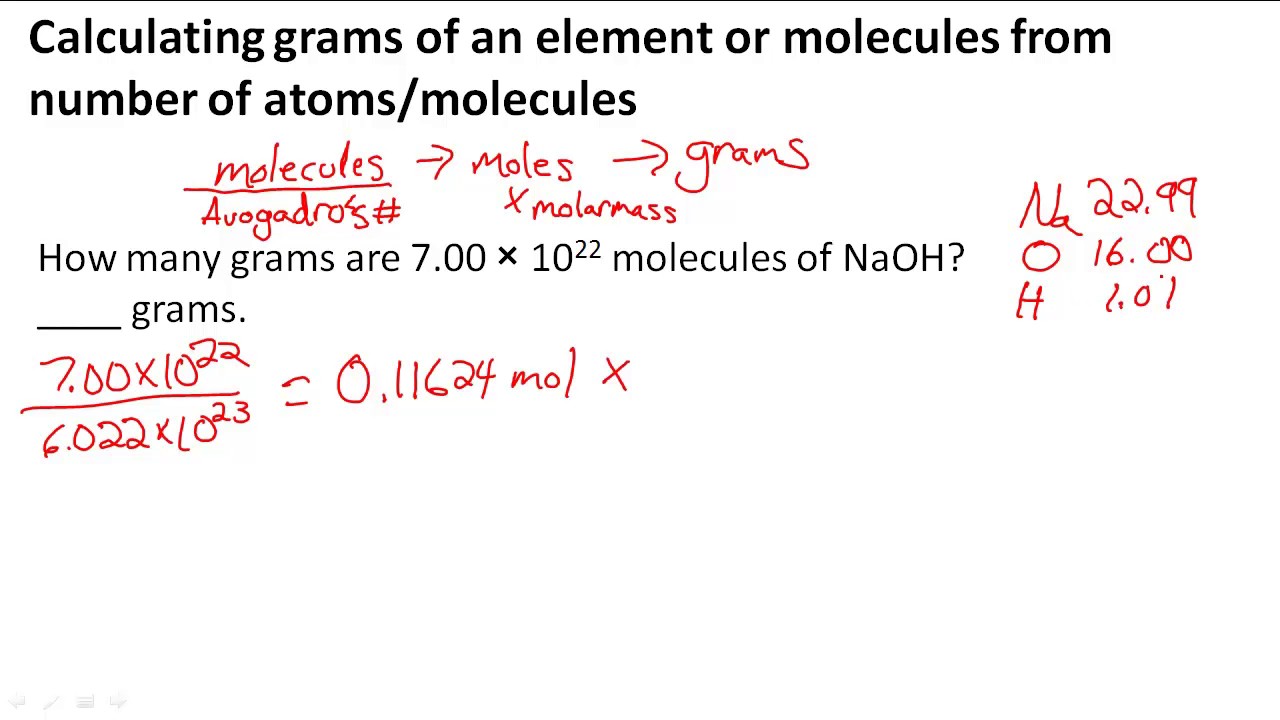 Source: youtube.com
Source: youtube.com
Thus the number of moles in the given sample of NaCl comes out to. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. 9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion. For example if you have 170 mol of NaCl then. Since you need to find for 360 mol of H.
 Source: pinterest.com
Source: pinterest.com
Multiply the elements atomic mass by the number of atoms of that element in the compound. For example imagine you have 2 g of water or H 2 O and you want to convert it to molesThe molecular mass of H 2 O is 18gmol. N the number of moles of a compound. 1 day ago One mole consists of Avogadro number of atomsIf you know the quantity of mole it can be. Look for the atomic masses of hydrogen sulfur and oxygen.
 Source: pinterest.com
Source: pinterest.com
1 day ago One mole consists of Avogadro number of atomsIf you know the quantity of mole it can be. To go from grams to moles divide the grams by the molar mass. The unit is typically gmol. Thus the number of moles in the given sample of NaCl comes out to. Hence one mole of H 2 SO 4 weights 106076 grams.
 Source: study.com
Source: study.com
The unit is typically gmol. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula. Calculate Moles of Product. Hence one mole of H 2 SO 4 weights 106076 grams. Im told these are incorrect answers.
 Source: youtube.com
Source: youtube.com
170 mol 58443 g 1 mol 994 g. Therefore there are 500 g 1011 gmol 00495 mol of KNO3. 600 g 58443 gmol 1027 mol of NaCl. 9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion. The quotient tells you the number of moles.
 Source: pinterest.com
Source: pinterest.com
The unit is typically gmol. For example imagine you have 2 g of water or H 2 O and you want to convert it to molesThe molecular mass of H 2 O is 18gmol. Thus the number of moles in the given sample of NaCl comes out to. 1 day ago One mole consists of Avogadro number of atomsIf you know the quantity of mole it can be. Multiply that by Avogadros number and youll find out how many atoms the sample contains.
 Source: study.com
Source: study.com
It refers to a huge number that we use to measure atoms. How many grams are in 0572 moles of glucose C 6 H 12 O 6. Since you need to find for 360 mol of H. 170 mol 58443 g 1 mol 994 g. Therefore there are 500 g 1011 gmol 00495 mol of KNO3.
 Source: pinterest.com
Source: pinterest.com
Calculate Moles of Product. Multiply the elements atomic mass by the number of atoms of that element in the compound. For example imagine you have 2 g of water or H 2 O and you want to convert it to molesThe molecular mass of H 2 O is 18gmol. This will give you the relative amount that each element contributes to the. Using a calculator divide the number of grams by the molar mass.
 Source: slideplayer.com
Source: slideplayer.com
How many grams are in 379 moles of calcium bromide CaBr 2. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula. Then 1000 g 151001 gmol. Determine the moles of product produced by dividing the grams of product by the grams per mole of product. To go from grams to moles divide the grams by the molar mass.
 Source: pinterest.com
Source: pinterest.com
Number of moles Weight of compound in grams molecular weight of compound. Hence one mole of H 2 SO 4 weights 106076 grams. The unit is typically gmol. Thus the number of moles in the given sample of NaCl comes out to. Since you need to find for 360 mol of H.
 Source: pinterest.com
Source: pinterest.com
Hence one mole of H 2 SO 4 weights 106076 grams. Ask me questions on Facebook. Therefore there are 500 g 1011 gmol 00495 mol of KNO3. But wait what actually is a mole. Look for the atomic masses of hydrogen sulfur and oxygen.
 Source: khanacademy.org
Source: khanacademy.org
Number of moles Weight of compound in grams molecular weight of compound. If you have a sample that contains only atoms of a particular element weigh the sample in grams and divide by the atomic weight of the element. N 1 mole of N for 1 mole of KNO3. Fifth convert to grams. Multiply that by Avogadros number and youll find out how many atoms the sample contains.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how do you find the number of grams per mole for an element by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






