Your How do you find the moles of one element in a compound images are ready. How do you find the moles of one element in a compound are a topic that is being searched for and liked by netizens today. You can Download the How do you find the moles of one element in a compound files here. Get all free photos and vectors.
If you’re searching for how do you find the moles of one element in a compound pictures information related to the how do you find the moles of one element in a compound topic, you have visit the ideal site. Our site frequently provides you with hints for viewing the maximum quality video and image content, please kindly search and find more enlightening video articles and graphics that fit your interests.
How Do You Find The Moles Of One Element In A Compound. 3 moles Nitrogen to grams 420201 grams. 5 moles Nitrogen to grams 700335 grams. Anything in the lab. You can view more details on each measurement unit.
 How To Convert From Moles Of One Substance To Moles Of Another Substance Stoichiometry Part 1 Youtube From youtube.com
How To Convert From Moles Of One Substance To Moles Of Another Substance Stoichiometry Part 1 Youtube From youtube.com
The atomic mass of carbon is 120 amu rounding to one decimal place and that of chlorine is 355 amu so one mole of carbon tetrachloride weighs 154 grams. 1 gram of mole is the amount of the molecules used to represent the moles of the molecules that are present in one mole of the substance. 602 x 1023 of them. If you are given the molarity of any solution and asked to calculate the number of moles simply multiply the molarity with the total volume of the solution in litres. If you do permit this you should make it very clear that you are using nonstandard lab materials and that this is not a. If there is no subscript it means there is only one atom of that element in the formula.
7 moles Nitrogen to grams 980469 grams.
This is defined as 0001 kilogram per mole or 1 gram per mole. You can find the moles of any mass of any compound. 6 moles Nitrogen to grams 840402 grams. 1 molecule of compound X a Y b contains. The mass of one mole of a compound is called its molar weight or molar mass. This gives you the mass in grams of one mole of the molecule.
 Source: youtube.com
Source: youtube.com
Multiply the number of atoms of an element by its atomic. If there is no subscript it means there is only one atom of that element in the formula. For a compound with the molecular formula X a Y b. The complete Lewis electron-dot. The first element of the periodic table is Hydrogen having mass number 101 and the last element of the periodic table is Ununoctium having mass number 294.
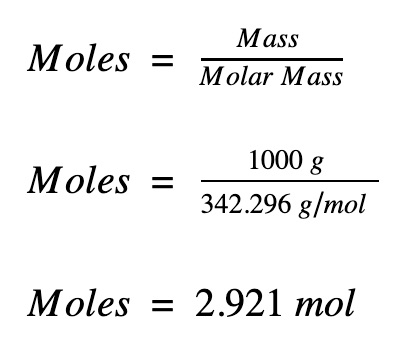 Source: surfguppy.com
Source: surfguppy.com
Pay attention to significant figures. Doing one or more moles lab activities in each unit you teach will give students plenty of practice. You can view more details on each measurement unit. Then add all of your answers together to find the molar mass of the compound. One mole equals to a very large number of particles.
 Source: slideplayer.com
Source: slideplayer.com
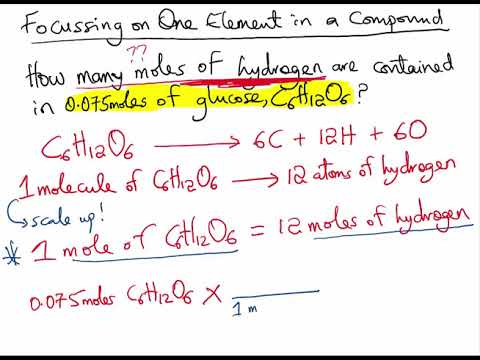
1 gram of mole is the amount of the molecules used to represent the moles of the molecules that are present in one mole of the substance. To calculate the molar mass you need to identify how many molecules of an element are in the compound and the atomic mass of each element in the compound. To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compound. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. A atoms of element X.
 Source: schoolbag.info
Source: schoolbag.info
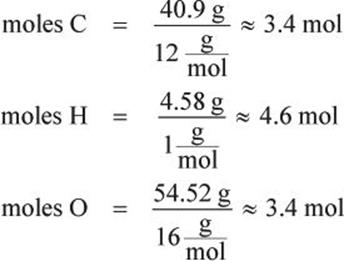
To do this look up atomic masses on the periodic table and use the formula mass to know how many atoms of each element are in a compound. 10 moles Nitrogen to grams 140067 grams. It allows you to easily convert between grams and moles of a substance. 3 moles Nitrogen to grams 420201 grams. 9 moles Nitrogen to grams 1260603 grams.
 Source: chem.fsu.edu
Source: chem.fsu.edu
You can view more details on each measurement unit. If there is no subscript it means there is only one atom of that element in the formula. 10 moles Nitrogen to grams 140067 grams. You look up the atomic mass of each of the elements in the formula multiply it by the number of atoms of that element in the compound and add it to all the others. A moles of.
 Source: youtube.com
Source: youtube.com
The compound urea H 2 NCONH 2 is widely used in chemical fertilizers. 3 moles Nitrogen to grams 420201 grams. A atoms of element X. 1 mole is equal to 1 moles NaOH or 3999711 grams. You can find the moles of any mass of any compound.
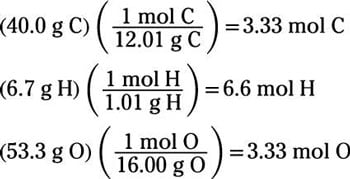 Source: dummies.com
Source: dummies.com
A moles of. The mass in grams of one mole of a compound is equal to the molecular weight of the compound in atomic mass units. Molecular weight of NaOH or grams This compound is also known as Sodium Hydroxide. B atoms of element Y. If there is no subscript it means there is only one atom of that element in the formula.
 Source: youtube.com
Source: youtube.com
A moles of. Then add all of your answers together to find the molar mass of the compound. Pay attention to significant figures. If you do permit this you should make it very clear that you are using nonstandard lab materials and that this is not a. 1 mole of compound X a Y b contains.
 Source: khanacademy.org
Source: khanacademy.org
In chemistry courses youll frequently have to convert moles to molecules or molecules to moles using Avogadros number. The atomic mass of carbon is 120 amu rounding to one decimal place and that of chlorine is 355 amu so one mole of carbon tetrachloride weighs 154 grams. 5 moles Nitrogen to grams 700335 grams. Anything in the lab. We assume you are converting between moles NaOH and gram.

One mole equals to a very large number of particles. Doing one or more moles lab activities in each unit you teach will give students plenty of practice. You can view more details on each measurement unit. 3 moles Nitrogen to grams 420201 grams. It allows you to easily convert between grams and moles of a substance.
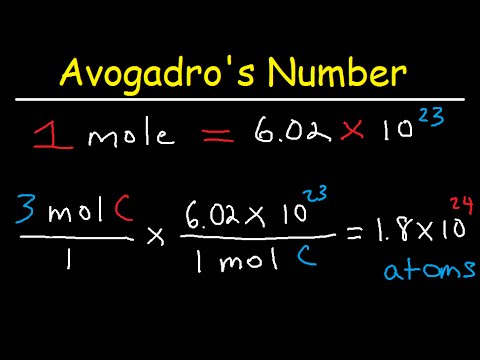 Source: youtube.com
Source: youtube.com
You look up the atomic mass of each of the elements in the formula multiply it by the number of atoms of that element in the compound and add it to all the others. 1 gram of mole is the amount of the molecules used to represent the moles of the molecules that are present in one mole of the substance. 602 x 1023 of them. This gives you the mass in grams of one mole of the molecule. 4 moles Nitrogen to grams 560268 grams.
 Source: khanacademy.org
Source: khanacademy.org
If you are given the molarity of any solution and asked to calculate the number of moles simply multiply the molarity with the total volume of the solution in litres. The molar mass is the amount in grams g of one mole of a compound. It allows you to easily convert between grams and moles of a substance. This is defined as 0001 kilogram per mole or 1 gram per mole. 602 x 1023 of them.
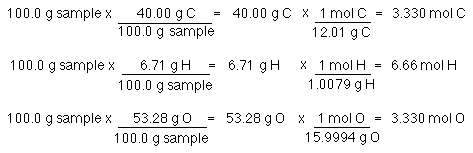 Source: westfield.ma.edu
Source: westfield.ma.edu
To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compound. Anything in the lab. 4 moles Nitrogen to grams 560268 grams. B atoms of element Y. 8 moles Nitrogen to grams 1120536 grams.
 Source: slideshare.net
Source: slideshare.net
3 moles Nitrogen to grams 420201 grams. If there is no subscript it means there is only one atom of that element in the formula. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. If you do permit this you should make it very clear that you are using nonstandard lab materials and that this is not a. First you have to convert the masses to the number of moles of each compound and to do that you have to look up the atomic masses of each of the elements in the periodic table.
 Source: youtube.com
Source: youtube.com
If you are given the molarity of any solution and asked to calculate the number of moles simply multiply the molarity with the total volume of the solution in litres. B atoms of element Y. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. Molecular weight formula helps the molecular mass calculator to find molar mass grams per moles and molecular weight.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how do you find the moles of one element in a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






