Your How do you find moles per liter images are ready in this website. How do you find moles per liter are a topic that is being searched for and liked by netizens today. You can Get the How do you find moles per liter files here. Download all royalty-free photos and vectors.
If you’re searching for how do you find moles per liter images information related to the how do you find moles per liter interest, you have visit the ideal blog. Our site frequently provides you with suggestions for seeing the maximum quality video and image content, please kindly surf and locate more enlightening video articles and graphics that match your interests.
How Do You Find Moles Per Liter. MolarityMoles of the soluteVolume of the solution555515555 M. Youll need to know the volume of water used. Moles mol Molarity M x Volume L 05 x 2. Molarity is the number of moles of a substance in one litre of solution.
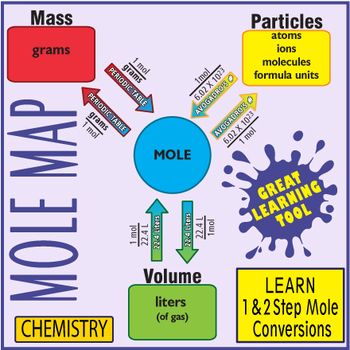 My Chemistry Students Find Using This Map The Easiest Way To Quickly Master 1 And 2 Step Mole Conver Teaching Chemistry Chemistry Education Chemistry Classroom From pinterest.com
My Chemistry Students Find Using This Map The Easiest Way To Quickly Master 1 And 2 Step Mole Conver Teaching Chemistry Chemistry Education Chemistry Classroom From pinterest.com
A step-by-step explanation of how to convert from liters to molesIn this video were converting from liters to moles. Molarity is the number of moles of a substance in one litre of solution. Moles mol Molarity M x Volume L 05 x 2. The SI derived unit for amount-of-substance concentration is the molecubic meter. N M V. Molar volume wikipedia.
Divide the mass by the molar mass to get the number of moles.
Now we know that the volume of the solution is 1 L the molarity of the solution is calculated by. We can rearrange this equation to get the number of moles. A 01 M solution contains one mole of solute per liter of solution. The SI derived unit for amount-of-substance concentration is the molecubic meter. For NaCl the molar mass is 5844 gmol. If you have a solution you multiply the molarity by the volume in litres.
 Source: br.pinterest.com
Source: br.pinterest.com
Then by dividing the total number of moles by the total volume of the solution one can find the molarity of the solution molesliter. Now we can use the rearranged equation. How to ger concentration molL of a solution from the mass grams of salt dissolved in water. For NaCl the molar mass is 5844 gmol. There are two steps.
 Source: pinterest.com
Source: pinterest.com
Divide the mass by the molar mass to get the number of moles. Molarity and density are different ways of expressing essentially the same thing. Divide the number of moles by the number of liters. So the Molarity of pure water is 5555 M. Moles mol Molarity M x Volume L 05 x 2.
 Source: pinterest.com
Source: pinterest.com
Moles mol Molarity M x Volume L 05 x 2. Molarity is the concentration of a solution expressed as the number of moles of solute per litre of. Youll need to know the volume of water used. To get the number of moles per liter of water we divide the mass of 1L of water 1000g by the mass of a mole of water 18g mole. Mass g No.
 Source: pinterest.com
Source: pinterest.com
When youre preparing a volume other than one liter and the amount of solute isnt as obvious it can be calculated using moles molarity x volume in liters. A 01 M solution contains one mole of solute per liter of solution. Molarity and density are different ways of expressing essentially the same thing. Molarity is the number of moles of a substance in one litre of solution. The calculator below uses the formula to convert liters to moles and to convert moles to liters where is 22413962.
 Source: pinterest.com
Source: pinterest.com
When youre preparing a volume other than one liter and the amount of solute isnt as obvious it can be calculated using moles molarity x volume in liters. Click to see full answer. Also to do this you need to know the volume of the solution and how many solutes has been dissolved in the solution. M n V where n is the number of moles and V is the volume in litres. Now we can use the rearranged equation.
 Source: hu.pinterest.com
Source: hu.pinterest.com
Also to do this you need to know the volume of the solution and how many solutes has been dissolved in the solution. The key to the conversion is realizin. Total number of moles 1 mole 585 g 6 g 062 moles Now determine moles per liter of solution. Moles mol x Molar Mass gmol 1 x 5844. Molarity is the concentration of a solution expressed as the number of moles of solute per litre of.
 Source: pinterest.com
Source: pinterest.com
1000 18 moles. Convert 750 mL to liters. For NaCl the molar mass is 5844 gmol. In this case you need to prepare 1 liter 1000 ml so you need 01 moles of NaOH. How do you convert moles to liters per molarity.
 Source: pinterest.com
Source: pinterest.com
N M V. To convert mgmL to molarity requires converting milligrams to grams and then using the molar mass of the solute to find the total number of moles. Molar volume wikipedia. To get the number of moles per liter of water we divide the mass of 1L of water 1000g by the mass of a mole of water 18g mole. Molarity moles soluteLiter solution.
 Source: pinterest.com
Source: pinterest.com
The key to the conversion is realizin. 1 molecubic meter is equal to 0001 molar or 0001 mole per liter. Molarity moles soluteLiter solution. Molarity 015 moles of KMnO 4 075 L of solution. Mass g No.
 Source: pinterest.com
Source: pinterest.com
When youre preparing a volume other than one liter and the amount of solute isnt as obvious it can be calculated using moles molarity x volume in liters. The SI derived unit for amount-of-substance concentration is the molecubic meter. Moles mol Molarity M x Volume L 05 x 2. For NaCl the molar mass is 5844 gmol. The official symbol for molarity is c concentration but many people use the old symbol M.
 Source: pinterest.com
Source: pinterest.com
MolarityMoles of the soluteVolume of the solution555515555 M. We can rearrange this equation to get the number of moles. To convert mgmL to molarity requires converting milligrams to grams and then using the molar mass of the solute to find the total number of moles. In this case you need to prepare 1 liter 1000 ml so you need 01 moles of NaOH. A step-by-step explanation of how to convert from liters to molesIn this video were converting from liters to moles.
 Source: pinterest.com
Source: pinterest.com
Calculate the number of moles of solute presentmol NaOH150g NaOHx1 mol NaOH400 g NaOHmol NaOH0375 mol NaOH Calculate the number of liters of solution presentL soln225 mLx1 L0225 L soln1000 mL Divide the number of moles of solute by the number of liters of solutionM0375 mol. Molarity is also known as the molar concentration of a solution. Divide the number of moles by the number of liters. We can rearrange this equation to get the number of moles. Liters of solution mL of solution x 1 L1000 mL Liters of solution 750 mL x 1 L1000 mL Liters of solution 075 L.
 Source: pinterest.com
Source: pinterest.com
Moles mol x Molar Mass gmol 1 x 5844. Molarity and density are different ways of expressing essentially the same thing. To convert mgmL to molarity requires converting milligrams to grams and then using the molar mass of the solute to find the total number of moles. A 01 M solution contains one mole of solute per liter of solution. How to ger concentration molL of a solution from the mass grams of salt dissolved in water.
 Source: pinterest.com
Source: pinterest.com
Molarity 015 moles of KMnO 4 075 L of solution. M n V where n is the number of moles and V is the volume in litres. For NaCl the molar mass is 5844 gmol. Liters of solution mL of solution x 1 L1000 mL Liters of solution 750 mL x 1 L1000 mL Liters of solution 075 L. 1 molecubic meter is equal to 0001 molar or 0001 mole per liter.
 Source: pinterest.com
Source: pinterest.com
How to ger concentration molL of a solution from the mass grams of salt dissolved in water. Click to see full answer. Molarity and density are different ways of expressing essentially the same thing. What is meant by 01 M solution. 1000 18 moles.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how do you find moles per liter by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






