Your How do you find moles from volume images are available in this site. How do you find moles from volume are a topic that is being searched for and liked by netizens now. You can Download the How do you find moles from volume files here. Find and Download all royalty-free images.
If you’re searching for how do you find moles from volume pictures information related to the how do you find moles from volume keyword, you have visit the ideal site. Our website always provides you with suggestions for seeing the maximum quality video and image content, please kindly surf and locate more informative video content and images that fit your interests.
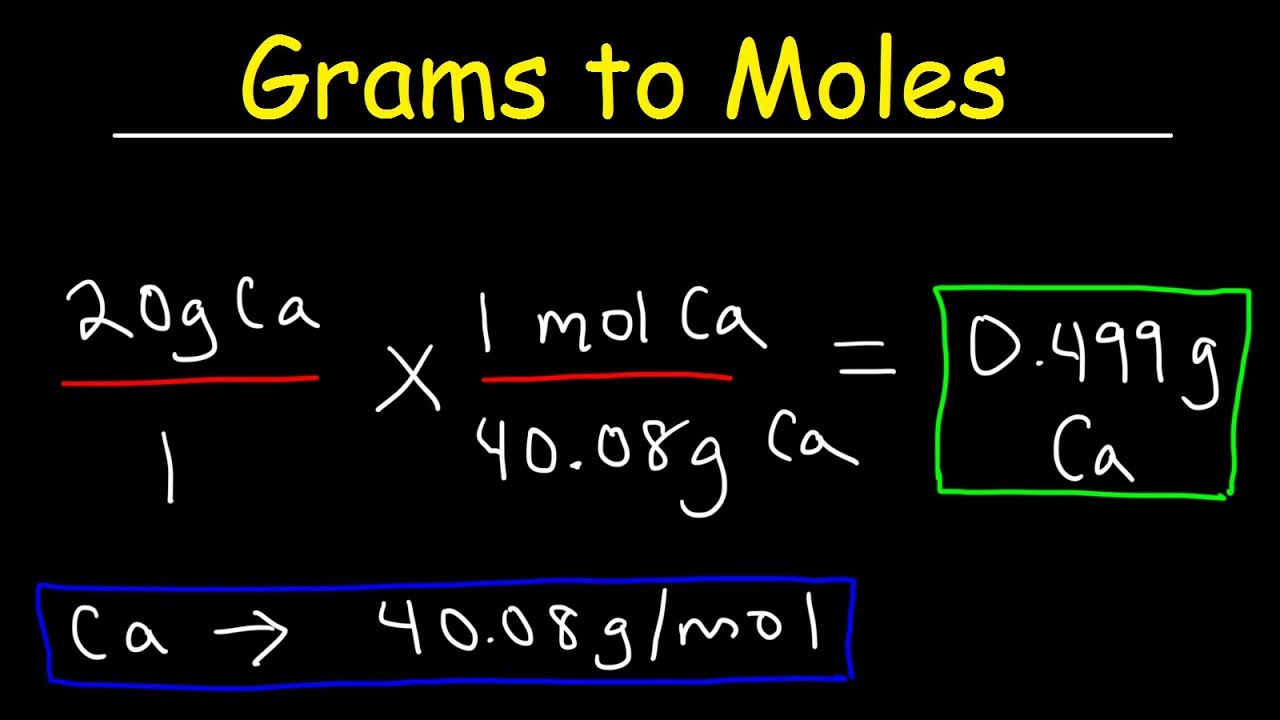
How Do You Find Moles From Volume. Standard temperature and pressure STP means temperature of 0oC and a pressure of 1013 kPa or 1 atmosphere atm. Gas volumes from moles and grams. Moles HI 00135 x 73 x 10-2 9855 x 10-4 moles Moles LiOH 00305 x 77 x 10-2 23485 x 10-3 moles. If you have a solution you multiply the molarity by the volume in litres.
 Moles Of Gases The Science And Maths Zone In 2021 Relative Atomic Mass Math How To Find Out From pinterest.com
Moles Of Gases The Science And Maths Zone In 2021 Relative Atomic Mass Math How To Find Out From pinterest.com
Molar volume of gases. In one mole of a substance there are 6022 x 10 23 particles. Number of moles volume of gas at rtp 24. Gas volumes from moles and grams. To calculate molarity divide the number of moles of solute by the volume of the solution in litersIf you dont know the number of moles of solute but you know the mass start by finding the molar mass of the solute which is equal to all of the molar masses of each element in the solution added together. It can be calculated by dividing the molar mass M by mass density ρ.
MOLES FROM VOLUME OF PURE LIQUID OR SOLID Multiply the volume by the density to get the mass.
08012014 Molarity is the number of moles of a substance in one litre of solution. In one mole of a substance there are 6022 x 10 23 particles. At standard Temperature and Pressure STP the molar volume Vm is the volume occupied by one mole of a chemical element or a chemical compound. Molar gas volume is one mole of any gas at a specific temperature and pressure has a fixed volume. At standard Temperature and Pressure STP the molar volume Vm is the volume occupied by one mole of a chemical element or a chemical compound. First of all before you can use this equation you need to know how many moles of solute are there in the solution.
 Source: pinterest.com
Source: pinterest.com
Calculate the volume of carbon dioxide gas CO 2 occupied by a 5 moles and b 05 moles of the gas occupied at STP. A Volume of CO 2 number of moles of CO 2 224 L 5 224. Molar gas volume is one mole of any gas at a specific temperature and pressure has a fixed volume. How to Calculate moles based on molarity and volume. You can rearrange this equation to solve the problem that is given in the question.
 Source: pinterest.com
Source: pinterest.com
Calculate the volume of carbon dioxide gas CO 2 occupied by a 5 moles and b 05 moles of the gas occupied at STP. Moles HI 00135 x 73 x 10-2 9855 x 10-4 moles Moles LiOH 00305 x 77 x 10-2 23485 x 10-3 moles. Calculate the volume of solution. It can be calculated by dividing the molar mass M by mass density ρ. Gas volumes from moles and grams.
 Source: pinterest.com
Source: pinterest.com
How to Calculate moles based on molarity and volume. In the example the amount of hydrogen is 202650 x 0025 29315 x 8314472 2078 moles. C 1 V 1 c 2 V 2 V 1 c 2 V 2 c 1 350 m o l l 1 750 m l 120 m o l l 1 21875 m l 219 m l. Volume 05 24 12 dm 3. This equation can be rearranged to find the amount of solute or volume of solution.
 Source: pinterest.com
Source: pinterest.com
A mole is defined as the number of substances containing particles of that substance as many as the atoms contained in 12000 grams of carbon atoms 12. A Volume of CO 2 number of moles of CO 2 224 L 5 224. At STP 1 mole or 602 x 1023 representative particles of any gas occupies a volume of 224 L. C 1 V 1 c 2 V 2 V 1 c 2 V 2 c 1 350 m o l l 1 750 m l 120 m o l l 1 21875 m l 219 m l. You can rearrange this equation to solve the problem that is given in the question.
 Source: pinterest.com
Source: pinterest.com
At STP 1 mole or 602 x 1023 representative particles of any gas occupies a volume of 224 L. MOLES FROM VOLUME OF PURE LIQUID OR SOLID Multiply the volume by the density to get the mass. It can be calculated by dividing the molar mass M by mass density ρ. How to Calculate moles based on molarity and volume. How do you find the number of moles given volume pressure and temperature.
 Source: pinterest.com
Source: pinterest.com
C 1 V 1 c 2 V 2 V 1 c 2 V 2 c 1 350 m o l l 1 750 m l 120 m o l l 1 21875 m l 219 m l. Volume 05 24 12 dm 3 Remember that 1 dm 3 1 000 cm 3 so the volume is also 12 000 cm 3 The equation can be rearranged to find the number of moles if. How do you find moles with pressure volume and temperature. Molar gas volume is one mole of any gas at a specific temperature and pressure has a fixed volume. How do you find the number of moles given volume pressure and temperature.
 Source: pinterest.com
Source: pinterest.com
You can rearrange this equation to solve the problem that is given in the question. Calculate the number of moles of solute. How do you find moles from volume. Calculate the molar mass of the solute. How do you find moles when given the volume of a gas.
 Source: pinterest.com
Source: pinterest.com
LiOH HI — LiI H2O 1mol 1 mol So you have excess LiOH 9855 x 10-4 moles of HI will react with 9855 x 10-4 moles of LiOH and leave 23485 x 10-3 moles - 9855 x 10-4 moles 1363 x 10-3 moles of excess LiOH. How to Calculate moles based on molarity and volume. And thus using the above-mentioned equation. Divide the mass by the molar mass to get the number of moles. Calculate the number of moles of solute.
 Source: pinterest.com
Source: pinterest.com
Molar gas volume is one mole of any gas at a specific temperature and pressure has a fixed volume. It can be calculated by dividing the molar mass M by mass density ρ. A Volume of CO 2 number of moles of CO 2 224 L 5 224. Remember that 1 dm 3 1 000 cm 3 so the volume is also 12 000 cm 3. You can rearrange this equation to solve the problem that is given in the question.
 Source: pinterest.com
Source: pinterest.com
How do you find moles with pressure volume and temperature. It can be calculated by dividing the molar mass M by mass density ρ. A Volume of CO 2 number of moles of CO 2 224 L 5 224. Molar gas volume is one mole of any gas at a specific temperature and pressure has a fixed volume. How to convert an amount in moles to a volume in mL.
 Source: pinterest.com
Source: pinterest.com
MOLES FROM VOLUME OF PURE LIQUID OR SOLID Multiply the volume by the density to get the mass. The equation can be rearranged to find the number of moles if the volume of gas at rtp is known. Volume 05 24 12 dm 3 Remember that 1 dm 3 1 000 cm 3 so the volume is also 12 000 cm 3 The equation can be rearranged to find the number of moles if. Volume 05 24 12 dm 3. STP Volume of gas and temperature are usually measured at STP.
 Source: pinterest.com
Source: pinterest.com
To calculate molarity divide the number of moles of solute by the volume of the solution in litersIf you dont know the number of moles of solute but you know the mass start by finding the molar mass of the solute which is equal to all of the molar masses of each element in the solution added together. At standard Temperature and Pressure STP the molar volume Vm is the volume occupied by one mole of a chemical element or a chemical compound. Standard temperature and pressure STP means temperature of 0oC and a pressure of 1013 kPa or 1 atmosphere atm. Calculate the molar mass of the solute. Divide the mass by the molar mass to get the number of moles.
 Source: pinterest.com
Source: pinterest.com
You can rearrange this equation to solve the problem that is given in the question. How do you find the number of moles given volume pressure and temperature. The equation can be rearranged to find the number of moles if the volume of gas at rtp is known. Again if the volume is in cm3 divide the volume by 1000 first to convert it into dm3. How do you find moles when given the volume of a gas.
 Source: pinterest.com
Source: pinterest.com
Again if the volume is in cm3 divide the volume by 1000 first to convert it into dm3. Divide the mass by the molar mass to get the number of moles. Divide the mass by the molar mass to get the number of moles. To find the volume of a solution divide the moles by the concentration in mol dm3. Calculate the molar mass of the solute.
 Source: pinterest.com
Source: pinterest.com
How to Calculate moles based on molarity and volume. Divide the mass by the molar mass to get the number of moles. To calculate molarity divide the number of moles of solute by the volume of the solution in litersIf you dont know the number of moles of solute but you know the mass start by finding the molar mass of the solute which is equal to all of the molar masses of each element in the solution added together. MOLES FROM VOLUME OF PURE LIQUID OR SOLID Multiply the volume by the density to get the mass. Where the value of 6022 x 10 23 particles per mole is called the Avogadro.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how do you find moles from volume by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.