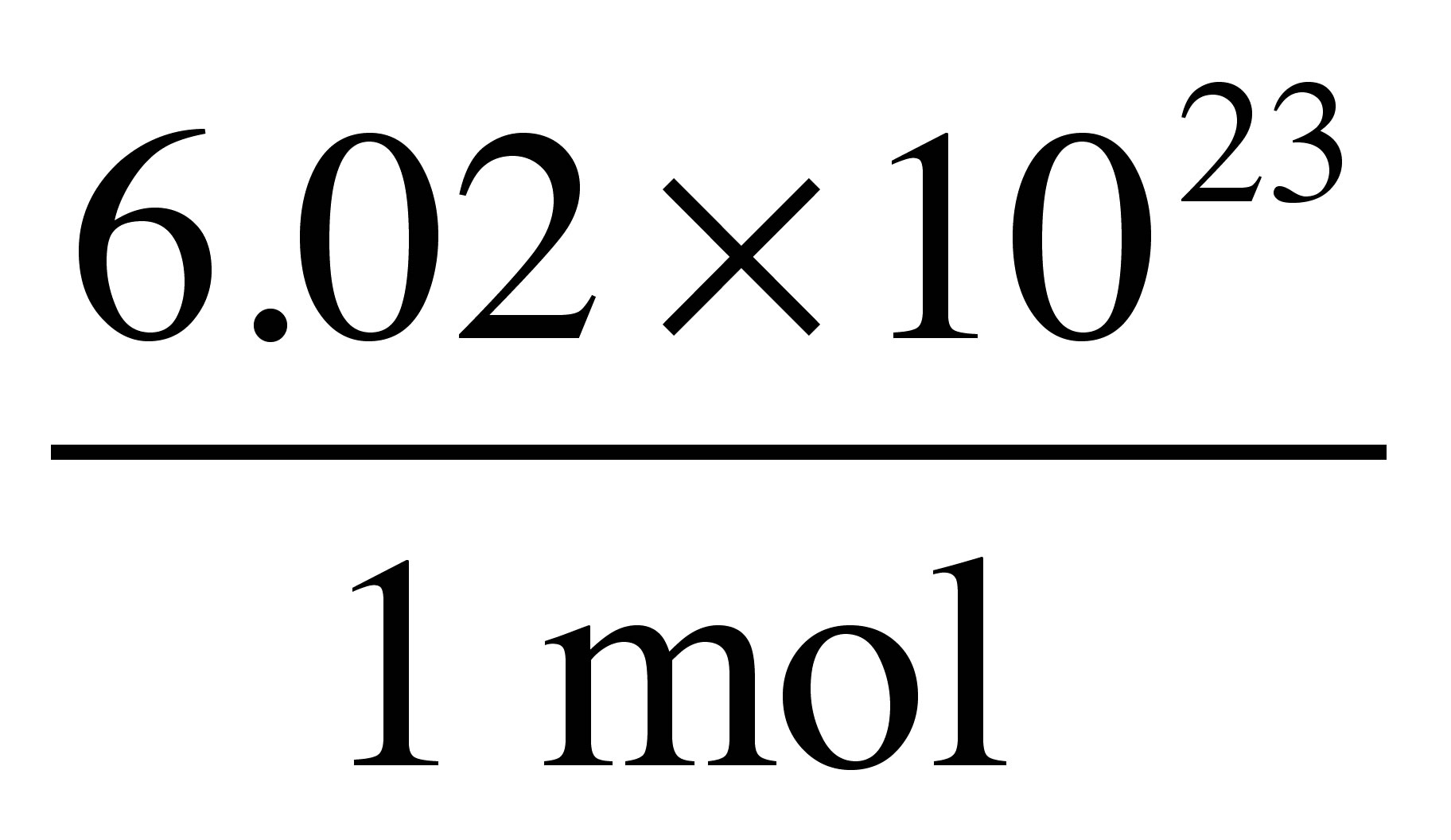Your How do you do moles to grams images are ready. How do you do moles to grams are a topic that is being searched for and liked by netizens today. You can Find and Download the How do you do moles to grams files here. Get all royalty-free images.
If you’re searching for how do you do moles to grams pictures information related to the how do you do moles to grams topic, you have pay a visit to the ideal blog. Our site always provides you with hints for downloading the maximum quality video and image content, please kindly search and find more informative video articles and graphics that fit your interests.
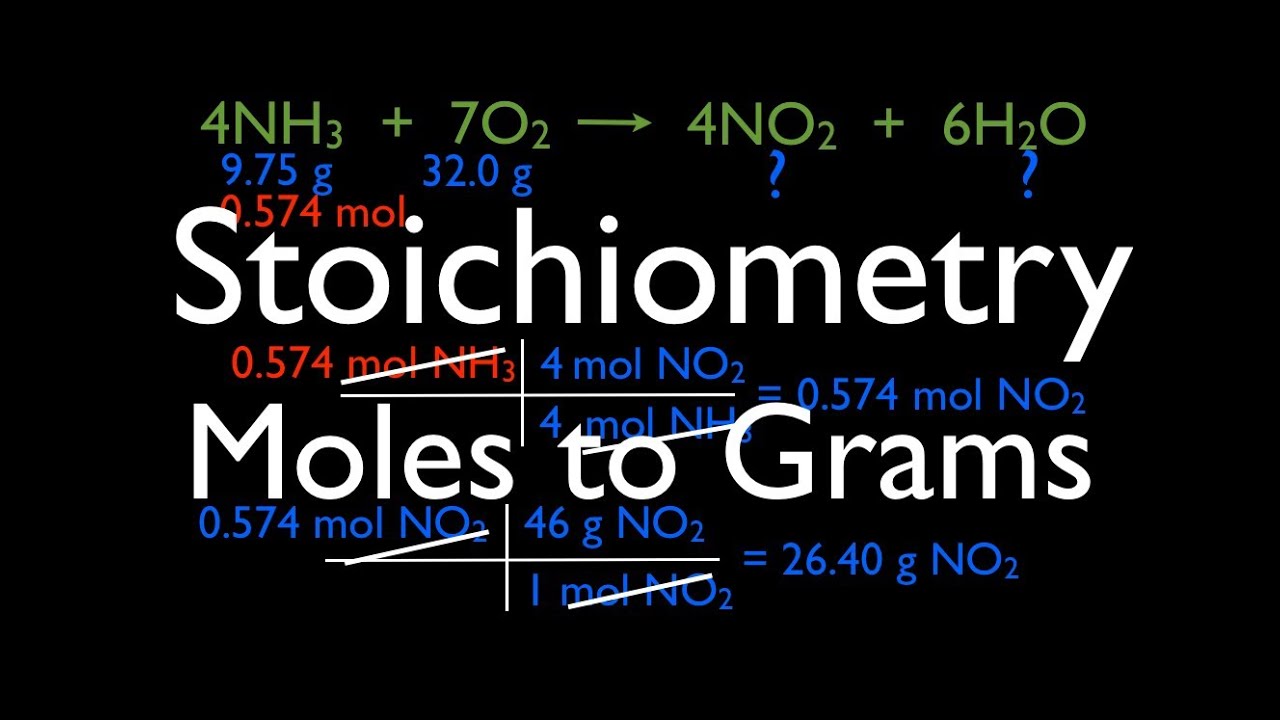
How Do You Do Moles To Grams. How do you calculate moles from grams. So to get mgmL from mmolL. This chemistry video tutorial explains find out how to convert the unit grams to moles which is a typical conversion step for a lot of stoichiometry questions. There are three steps to converting moles of a substance to grams.
 How To Convert Grams Moles Atoms And Liters In Chemistry In 2021 School Organization Notes School Study Tips Chemistry Quotes From pinterest.com
How To Convert Grams Moles Atoms And Liters In Chemistry In 2021 School Organization Notes School Study Tips Chemistry Quotes From pinterest.com
So if one mole has a mass of 18 grams 25 grams would have a mass of 25 grams 18 grams per mole or 139 moles. How to Convert Grams to Moles. To find the number of moles in a solution multiply the concentration by the volume in dm3. To find the concentration of a solution divide the number of moles by the volume in dm3. 02 x 5844 11688 grams. Here are three important steps to follow to convert moles to grams.
You can also use our best moles grams calculator to determine the molecules or atoms present in grams.
N m M where M is the molar mass of this material. The formula for moles to grams is given by. Multiply step one by step two. Divide the number of grams of the substance by the molecular mass. How do you find moles from grams and molar mass. To find the concentration of a solution divide the number of moles by the volume in dm3.
 Source: pinterest.com
Source: pinterest.com
If the volume is in cm3 divide the volume by 1000 first to convert it into dm3. Hence one mole of H 2 SO 4 weights 106076 grams. Divide the number of grams of the substance by the molecular mass. 6 moles in to grams 688908 grams. The formula for moles to grams is given by.
 Source: pinterest.com
Source: pinterest.com
Multiply step one by step two. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. Look for the atomic masses of hydrogen sulfur and oxygen. Now calculate the molar mass of the substance. The grams cancel because they are both on the bottom of the equation if you make the amount in grams a fraction over 1 gram which leaves moles and the answer.
 Source: pinterest.com
Source: pinterest.com
So to convert from grams to moles divide the amount in grams by the atomic weight in grams to find the amount of moles. So one mole of water has a mass of 16 11 18 grams. Now calculate the molar mass of the substance. Multiply the number of moles by the molar mass to obtain the final answer in grams. You can do the reverse unit conversion from grams ga to moles or enter other units to convert below.
 Source: pinterest.com
Source: pinterest.com
Since you need to find for 360 mol of H. The answer will be the number of moles of the compound. First of all find the number of moles by using mass and molar mass. What is moles to grams. The number of moles present in a compound is often given to the student in the problem.
 Source: pinterest.com
Source: pinterest.com
9 moles co2 to grams 3960855 grams. Now calculate the molar mass of the substance. Multiply step one by step two. N m M where M is the molar mass of this material. If the volume is in cm3 divide the volume by 1000 first to convert it into dm3.
 Source: pinterest.com
Source: pinterest.com
See an example of converting grams to moles. The number of moles present in a compound is often given to the student in the problem. After about a semester and a half I had forgotten how to convert from grams to molesThis article was extremely clear precise and definitive. N 5988 g 18015 gmol 3324 mol. Grams Moles x Molar Mass.
 Source: pinterest.com
Source: pinterest.com
Convert 250 g of water to moles of water. You can also use our best moles grams calculator to determine the molecules or atoms present in grams. 02 x 5844 11688 grams. Convert 250 g of water to moles of water. 7 moles ga to grams 488061 grams.
 Source: pinterest.com
Source: pinterest.com
To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. This is equal to the number of grams in one mole of the substance. The three steps above can be expressed in the following ratio and proportion. Therefore the molecular mass of H 2 SO 4 is. First of all find the number of moles by using mass and molar mass.
 Source: pinterest.com
Source: pinterest.com
This is equal to the number of grams in one mole of the substance. See an example of converting grams to moles. Multiply the substances molar mass gmol by the number of moles. First of all find the number of moles by using mass and molar mass. Besides how do you convert moles to dm3.
 Source: hu.pinterest.com
Source: hu.pinterest.com
9 moles co2 to grams 3960855 grams. There are three steps to converting moles of a substance to grams. Formula to convert moles to grams. Determine the number of moles. 21008 3206 418 106076.
 Source: pinterest.com
Source: pinterest.com
Convert 250 g of water to moles of water. First of all you should check total number of moles given in the problem. More commonly written for this application as. Here are three important steps to follow to convert moles to grams. If it is not then it can be found by looking at the.
 Source: pinterest.com
Source: pinterest.com
First of all find the number of moles by using mass and molar mass. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions. 21008 3206 418 106076. Multiply step one by step two. So to convert from grams to moles divide the amount in grams by the atomic weight in grams to find the amount of moles.
 Source: pinterest.com
Source: pinterest.com
The three steps above can be expressed in the following ratio and proportion. Make sure you have a periodic table and a calculator handy. Hence one mole of H 2 SO 4 weights 106076 grams. Convert 02 moles of Sodium chloride. Multiply the variety of moles by the molar mass to acquire the ultimate reply in grams.
 Source: pinterest.com
Source: pinterest.com
Grams Moles x Molar Mass. 1 Gram-mole g-mol 1 000 Millimole mmol Measurement calculator that can be used to convert Gram-mole to Millimole among others. First of all you should check total number of moles given in the problem. How do you discover grams to moles. Therefore the molecular mass of H 2 SO 4 is.
 Source: pinterest.com
Source: pinterest.com
So if one mole has a mass of 18 grams 25 grams would have a mass of 25 grams 18 grams per mole or 139 moles. So if one mole has a mass of 18 grams 25 grams would have a mass of 25 grams 18 grams per mole or 139 moles. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. Where is the molar mass of the substance. The answer will be the number of moles of the compound.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how do you do moles to grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.