Your How do you determine the mass of 1 mole of a compound images are ready. How do you determine the mass of 1 mole of a compound are a topic that is being searched for and liked by netizens today. You can Download the How do you determine the mass of 1 mole of a compound files here. Find and Download all free photos and vectors.
If you’re searching for how do you determine the mass of 1 mole of a compound images information related to the how do you determine the mass of 1 mole of a compound interest, you have pay a visit to the ideal site. Our website frequently provides you with suggestions for downloading the highest quality video and image content, please kindly surf and locate more informative video content and images that match your interests.
How Do You Determine The Mass Of 1 Mole Of A Compound. Determine the moles of unknown the solute from the molarityof the solution and the volume in liters of the solution. In other words it tells you the number of. Also to know is how do you find the molar mass of an element. To find the molar mass of a compound.
 Mole Mass Atom Conversions Atom Chemistry Words From pinterest.com
Mole Mass Atom Conversions Atom Chemistry Words From pinterest.com
The molar mass of a substance is the mass in grams of 1 mole of the substance. N the number of moles of a compound. The molar mass is the mass of a given chemical element or chemical compound g divided by the amount of substance mol. Next multiply the number of a particular element by its molar mass. Multiply the atomic weight of each element with the number of atoms of that particular element. All you need to do is multiply the mass of each element in the formula by its subscript then add up all the results to get the mass of one mole of that compound.
Add up all the values obtained in the above step.
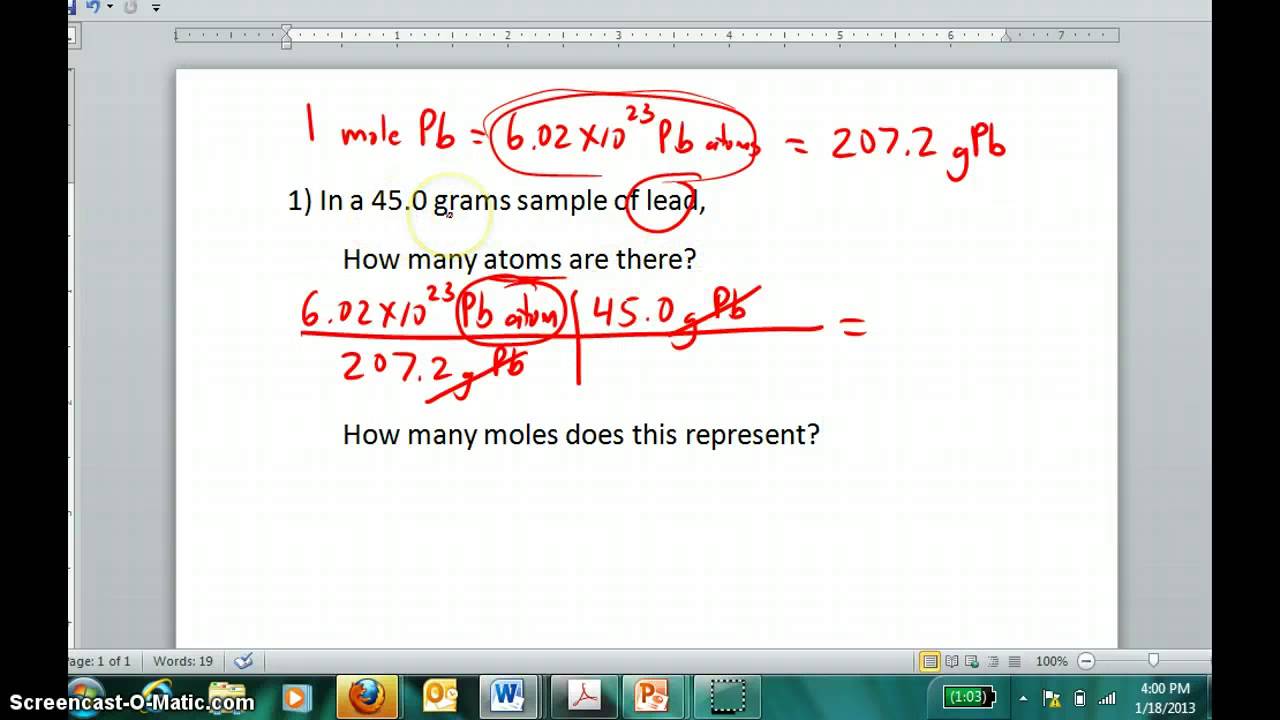
For example if you have 170. As shown in this video we can obtain a substances molar mass by summing the molar masses of its component atoms. Step 1 Determine the Molar Mass add molar masses of the individual elements Na and F both have 1 as subscript 2299g 18998g 41988g for NaF Step 2 Determine the Conversion Factor 325given of moles 325 mol Step 3 Solve 325 mol NaF 41988g NaF1 mole NaF 136461g NaF 325 moles of NaF 136 g of NaF How many molecules are in 325. Use the chemical formula to determine the number of each type of atom present in the compound. Moles of element n moles elementone mole of compound K -. The molar mass is the mass of a given chemical element or chemical compound g divided by the amount of substance mol.
 Source: khanacademy.org
Source: khanacademy.org
Determine the molar mass from the mass of the unknown and thenumber of moles of unknown. This determines the molar mass for the entire compound. Also to know is how do you find the molar mass of an element. Multiply the atomic weight of each element with the number of atoms of that particular element. This chemistry video tutorial explains how to calculate the molar mass of a compound.
 Source: pinterest.com
Source: pinterest.com
The molar mass of KNO3 is 391 140 3160 gmol 1011 gmol. Mole basically means the amount or mass of substance which contains the same units that 12 g of a C-12 atom has. This chemistry video tutorial explains how to calculate the molar mass of a compound. For example if you have 600 g of NaCl then 600 g58443 gmol 1027 mol of NaCl. Mass percent composition of K mass contribution of Kmolecular mass of K 3 Fe CN 6 x 100.
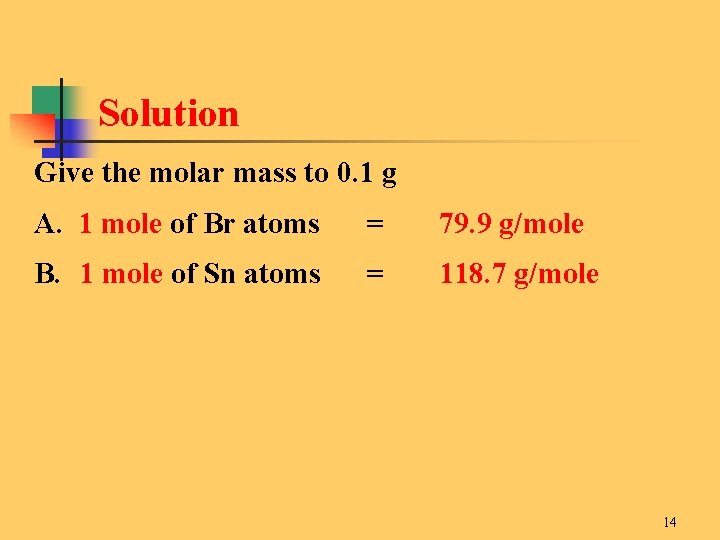 Source: slidetodoc.com
Source: slidetodoc.com
To convert between grams and moles you would use the substances molar mass. Multiply the atomic weight of each element with the number of atoms of that particular element. For example if you have 600 g of NaCl then 600 g58443 gmol 1027 mol of NaCl. This chemistry video tutorial explains how to calculate the molar mass of a compound. The molar mass is the mass of a given chemical element or chemical compound g divided by the amount of substance mol.
 Source: pinterest.com
Source: pinterest.com
To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3. Answer 1 of 3. Mole given weight molecular weight. Also to know is how do you find the molar mass of an element. Step 1 Determine the Molar Mass add molar masses of the individual elements Na and F both have 1 as subscript 2299g 18998g 41988g for NaF Step 2 Determine the Conversion Factor 325given of moles 325 mol Step 3 Solve 325 mol NaF 41988g NaF1 mole NaF 136461g NaF 325 moles of NaF 136 g of NaF How many molecules are in 325.
 Source: pinterest.com
Source: pinterest.com
Multiply the atomic weight of each element with the number of atoms of that particular element. Molarity moles of solute litres of solution. To go from grams to moles divide the grams by the molar mass. Multiply the atomic weight from the periodic table of each element by the number of atoms of that element present in the compound. M 9 12 g m o l 1 1 13 g m o l 1 14 g m o l 1 3 16 g m o l 1 183 g m o l 1.
 Source: pinterest.com
Source: pinterest.com
Molarity moles of solute litres of solution. N the number of moles of a compound. Therefore there are 500 g 1011 gmol 00495 mol of KNO3. As shown in this video we can obtain a substances molar mass by summing the molar masses of its component atoms. Determine the moles of unknown the solute from the molarityof the solution and the volume in liters of the solution.
 Source: slideplayer.com
Source: slideplayer.com
One mole of a compound contains Avogadros number 6022 x 10 23 of molecules molecular compound or formula units ionic compoundThe molar mass of a compound tells you the mass of 1 mole of that substance. This chemistry video tutorial explains how to calculate the molar mass of a compound. Now the molecular mass of carbon -12 atom in grams is 12 g. Created by Sal Khan. Mole basically means the amount or mass of substance which contains the same units that 12 g of a C-12 atom has.
 Source: youtube.com
Source: youtube.com
To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3. To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3. This would be the mass of 1 mol of K₂S. Now the molecular mass of carbon -12 atom in grams is 12 g. Click to see full answer.
 Source: chemteam.info
Source: chemteam.info
Mass percent composition of K mass contribution of Kmolecular mass of K 3 Fe CN 6 x 100. Mole basically means the amount or mass of substance which contains the same units that 12 g of a C-12 atom has. Determine the molar concentration of the unknown in the solutionfrom the observed osmotic pressure. Now to calculate its molar mass we add up all of the molar masses of each atom. Then add the unit as gramsmole you will get the molecular mass of the substance.
 Source: slideplayer.com
Source: slideplayer.com
Answer 1 of 3. Therefore there are 500 g 1011 gmol 00495 mol of KNO3. Finally add the products together and youll arrive at the answer. As shown in this video we can obtain a substances molar mass by summing the molar masses of its component atoms. For finding out this you have to multiply the mass of solute by its molar mass conversion factor.
 Source: slideplayer.com
Source: slideplayer.com
Step 1 Determine the Molar Mass add molar masses of the individual elements Na and F both have 1 as subscript 2299g 18998g 41988g for NaF Step 2 Determine the Conversion Factor 325given of moles 325 mol Step 3 Solve 325 mol NaF 41988g NaF1 mole NaF 136461g NaF 325 moles of NaF 136 g of NaF How many molecules are in 325. Add up all the values obtained in the above step. First of all before you can use this equation you need to know how many moles of solute are there in the solution. Then use the molality equation to calculate the moles of solute. In this compound there are 1 C 4 H 31 and 1 O.
 Source: pinterest.com
Source: pinterest.com
Start by determining how many of each elements there are by looking the subscripts small number next to the element symbol. To go from moles to grams multiply by the formula mass. We can then use the calculated molar mass to convert between mass and number of moles of the substance. Finally add the products together and youll arrive at the answer. Finding molar mass also called molecular weight molecular mass and gram formula mass is an essential skill in chemistry especially for mole to gram conv.
 Source: slideplayer.com
Source: slideplayer.com
Use the chemical formula to determine the number of each type of atom present in the compound. The molar mass of KNO3 is 391 140 3160 gmol 1011 gmol. Created by Sal Khan. To go from moles to grams multiply by the formula mass. To find the mass percent composition of an element divide the mass contribution of the element by the total molecular mass.
 Source: pinterest.com
Source: pinterest.com
For finding out this you have to multiply the mass of solute by its molar mass conversion factor. Also to know is how do you find the molar mass of an element. To go from moles to grams multiply by the formula mass. Start by determining how many of each elements there are by looking the subscripts small number next to the element symbol. Similarly do it for all the elements in the molecule or compound.
 Source: pinterest.com
Source: pinterest.com
This chemistry video tutorial explains how to calculate the molar mass of a compound. This number must then be multiplied by 100 to be expressed as a percent. Suppose that you have 500 g of KNO3. The molar mass is the mass of a given chemical element or chemical compound g divided by the amount of substance mol. All you need to do is multiply the mass of each element in the formula by its subscript then add up all the results to get the mass of one mole of that compound.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how do you determine the mass of 1 mole of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






