Your How do you convert molecular weight to grams images are ready in this website. How do you convert molecular weight to grams are a topic that is being searched for and liked by netizens now. You can Find and Download the How do you convert molecular weight to grams files here. Download all royalty-free photos and vectors.
If you’re searching for how do you convert molecular weight to grams pictures information related to the how do you convert molecular weight to grams topic, you have come to the ideal blog. Our site frequently gives you suggestions for viewing the highest quality video and picture content, please kindly search and locate more enlightening video content and graphics that match your interests.
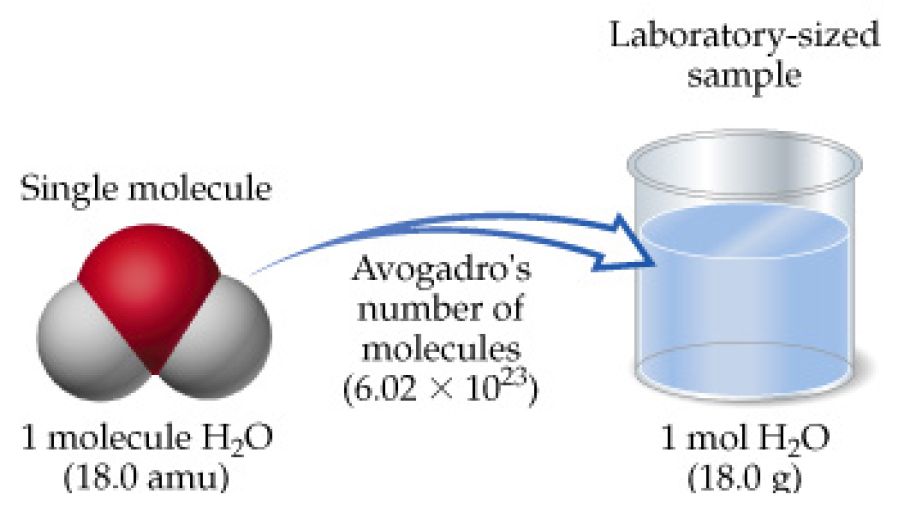
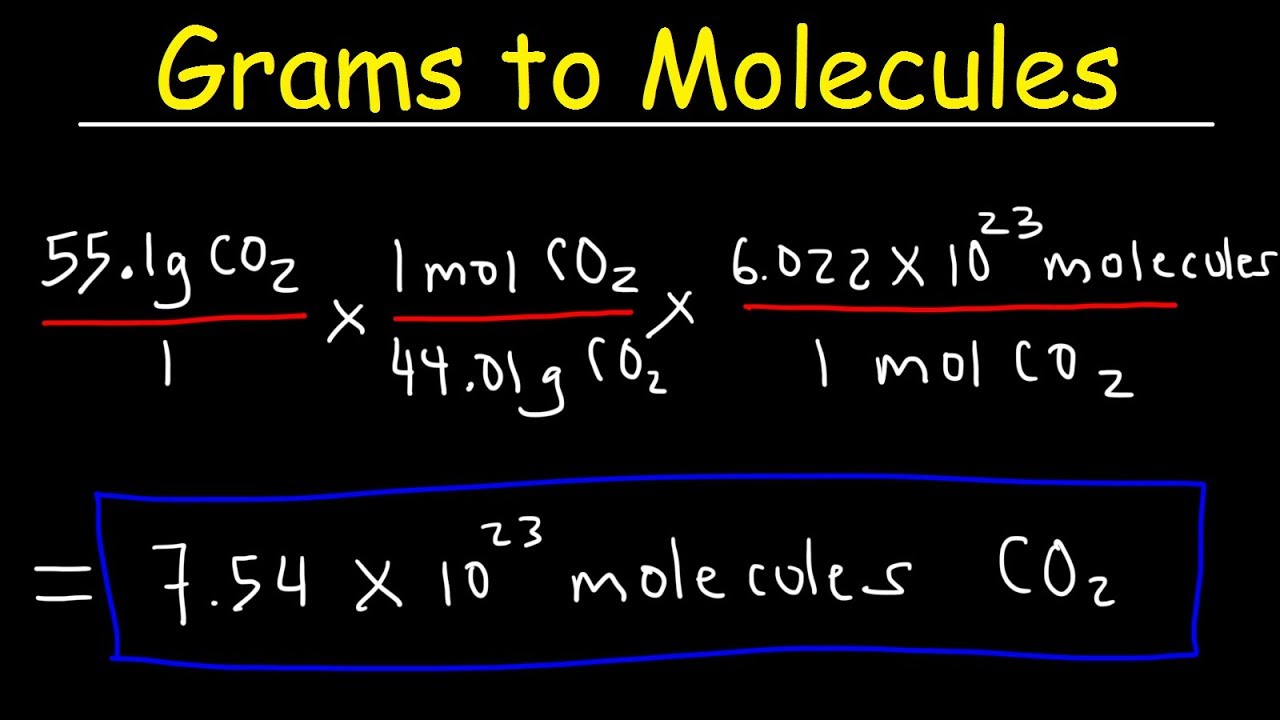
How Do You Convert Molecular Weight To Grams. Do a quick conversion. Calculate how many moles are mentioned in the question. To convert from molecules to grams it is necessary to first convertthe number of molecules of a substance by dividing by Avogadros number to find the number of moles and then multiply the number of moles by the molar mass of this substance. 1 moles co2 440095 gram using the molecular weight calculator and the molar mass of CO2.
 Grams To Moles Calculator Plus Convert Moles To Grams In 2021 Grams To Moles Molar Mass Mass To Moles From pinterest.com
Grams To Moles Calculator Plus Convert Moles To Grams In 2021 Grams To Moles Molar Mass Mass To Moles From pinterest.com
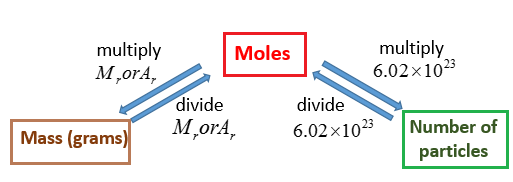
To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. Despite the very fact that the milliliter may be a unit of volume and gram a unit of weight its probable to convert over between the two supplying you with know the density of your material or ingredient. The SI base unit for amount of substance is the mole. Find the molar mass of the substance. Use the molar mass formula weight to convert from grams to moles or moles to grams. Molecular weight of Ag or grams The molecular formula for Ag is Ag.
We assume you are converting between moles Ag and gram.
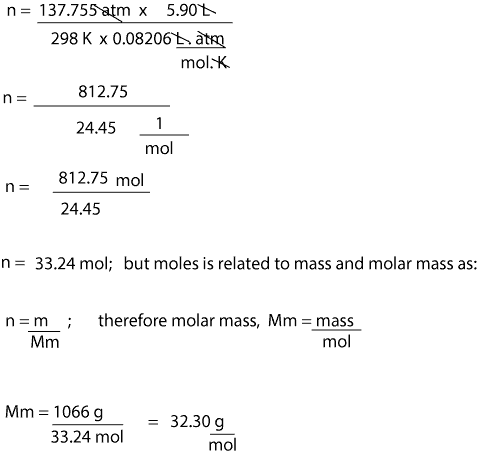
A mass in grams numerically equal to the molecular weight contains one mole of molecules which is known to be 602 x 1023 Avogadros number. If the MW 33325 Da then it has a molar mass of 33325 gmol or 33325 pounds per pound-mole. Find the molar mass of the substance. Calculate how many moles are mentioned in the question. The SI base unit for amount of substance is the mole. Do a quick conversion.
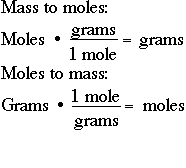 Source: socratic.org
Source: socratic.org
If playback doesnt begin shortly try. One molecule of molecular weight 33325 Da has a mass of 1219982 x 10-24 lb. Following video explain above statement. Do a quick conversion. Check the chart for more details.
 Source: clutchprep.com
Source: clutchprep.com
This is the weight in grams of 1 mole of the compound. Avogadros number is given as 6022 x 1023. If you know the quantity of mole it can be converted into grams and vice versa. To convert from molecules to grams it is necessary to first convertthe number of molecules of a substance by dividing by Avogadros number to find the number of moles and then multiply the number of moles by the molar mass of this substance. Do a quick conversion.
 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
One mole consists of Avogadro number of atoms. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. Molecular weight of Ag or grams The molecular formula for Ag is Ag. If the amount of the protein you purchased is 20 µg and the total volume is 100 µL 01 mL then this protein products. Convert 15 u to g.
 Source: wou.edu
Source: wou.edu
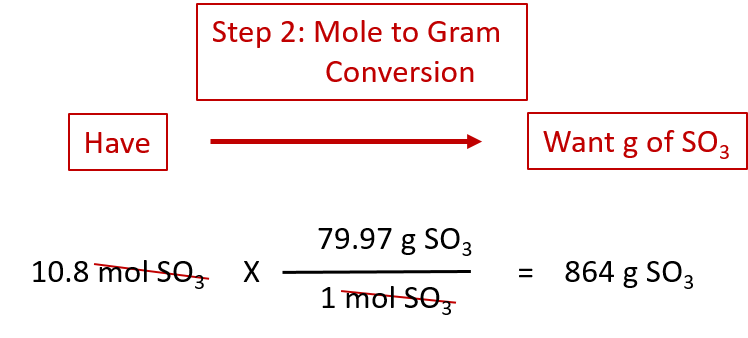
To do this youll want to be familiar with the definitions of both moles and molecules the relationship between the two concepts and the exact formula which uses Avogadros number. Find the molar mass of the substance. If playback doesnt begin shortly try. Molecular weight of Ag or grams The molecular formula for Ag is Ag. 1 mole is equal to 1 moles Ag or 1078682 grams.
 Source: wikihow.com
Source: wikihow.com
1 moles co2 440095 gram using the molecular weight calculator and the molar mass of CO2. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. This is the weight in grams of 1 mole of the compound. Conversions Table 30 Grams to Kilograms 003 1000 Grams to Kilograms 1 40 Grams to Kilograms 004 10000 Grams to Kilograms 10 50 Grams to Kilograms 005 100000 Grams to Kilograms 100 60 Grams to Kilograms 006 1000000 Grams to Kilograms 1000. If the amount of the protein you purchased is 20 µg and the total volume is 100 µL 01 mL then this protein products.
 Source: wikihow.com
Source: wikihow.com
Following video explain above statement. If playback doesnt begin shortly try. If you know the quantity of mole it can be converted into grams and vice versa. Avogadros number is given as 6022 x 1023. The SI base unit for amount of substance is the mole.
 Source: youtube.com
Source: youtube.com
1 moles co2 440095 gram using the molecular weight calculator and the molar mass of CO2. To convert from molecules to grams it is necessary to first convertthe number of molecules of a substance by dividing by Avogadros number to find the number of moles and then multiply the number of moles by the molar mass of this substance. Following video explain above statement. We can use this to get from a quantity expressed in grams like 74 grams of water a macroscopic quantity that we might weigh out in a lab to the quantity expressed in number of moles of water a molecular quantity. Grams Moles x Molar Mass.
 Source: khanacademy.org
Source: khanacademy.org
In chemistry courses youll frequently have to convert moles to molecules or molecules to moles using Avogadros number. Find the molar mass of the substance. The SI base unit for amount of substance is the mole. 15 u 15 16605402E-24 g 24908103E-23 g. You can view more details on each measurement unit.
 Source: wikihow.com
Source: wikihow.com
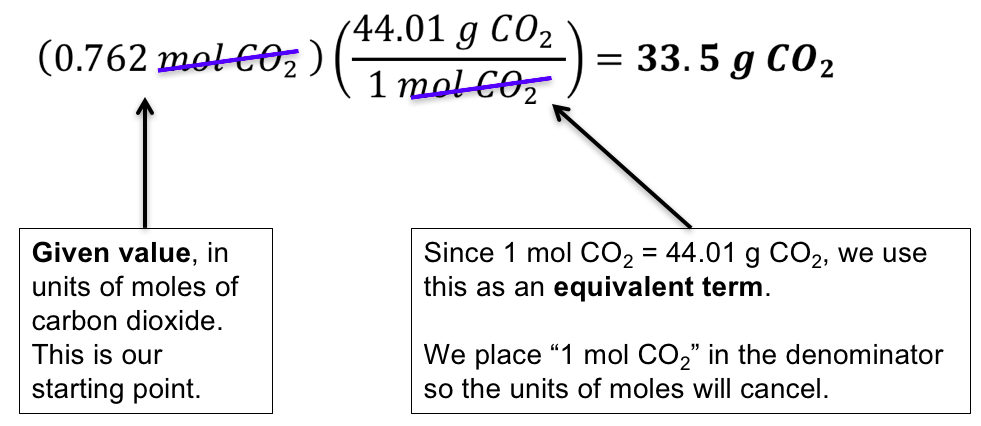
If the MW 33325 Da then it has a molar mass of 33325 gmol or 33325 pounds per pound-mole. Conversions Table 30 Grams to Kilograms 003 1000 Grams to Kilograms 1 40 Grams to Kilograms 004 10000 Grams to Kilograms 10 50 Grams to Kilograms 005 100000 Grams to Kilograms 100 60 Grams to Kilograms 006 1000000 Grams to Kilograms 1000. To do this youll want to be familiar with the definitions of both moles and molecules the relationship between the two concepts and the exact formula which uses Avogadros number. Use the molar mass formula weight to convert from grams to moles or moles to grams. It is much more common to use the amount of substance or number of moles.
 Source: pinterest.com
Source: pinterest.com
Despite the very fact that the milliliter may be a unit of volume and gram a unit of weight its probable to convert over between the two supplying you with know the density of your material or ingredient. The SI base unit for amount of substance is the mole. To do this we take our existing 74 grams water and multiply by our ratio of 1 mole of water per 18015 grams of water. One molecule of molecular weight 33325 Da has a mass of 1219982 x 10-24 lb. Find the molar mass of the substance.
 Source: studylib.net
Source: studylib.net
In chemistry courses youll frequently have to convert moles to molecules or molecules to moles using Avogadros number. Despite the very fact that the milliliter may be a unit of volume and gram a unit of weight its probable to convert over between the two supplying you with know the density of your material or ingredient. Conversions Table 30 Grams to Kilograms 003 1000 Grams to Kilograms 1 40 Grams to Kilograms 004 10000 Grams to Kilograms 10 50 Grams to Kilograms 005 100000 Grams to Kilograms 100 60 Grams to Kilograms 006 1000000 Grams to Kilograms 1000. A mass in grams numerically equal to the molecular weight contains one mole of molecules which is known to be 602 x 1023 Avogadros number. µM µgmL MW in KD nM ngmLMW in KD pM pgmL MW in KD.
 Source: youtube.com
Source: youtube.com
Avogadros number is given as 6022 x 1023. Converting Protein Mass Concentration to Molar Concentration Or Vice Versa From mass concentration to molar concentration. Check the chart for more details. Use this page to learn how to. Following video explain above statement.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
To convert from molecules to grams it is necessary to first convertthe number of molecules of a substance by dividing by Avogadros number to find the number of moles and then multiply the number of moles by the molar mass of this substance. In chemistry courses youll frequently have to convert moles to molecules or molecules to moles using Avogadros number. 2007 Joseph TE. A mass in grams numerically equal to the molecular weight contains one mole of molecules which is known to be 602 x 1023 Avogadros number. Formula to convert moles to grams.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
1 mole is equal to 1 moles Ag or 1078682 grams. Add the weight of each atom in the compound. Find the molar mass of the substance. This is the weight in grams of 1 mole of the compound. To do this youll want to be familiar with the definitions of both moles and molecules the relationship between the two concepts and the exact formula which uses Avogadros number.
 Source: study.com
Source: study.com
The simple formula is. Use this page to learn how to. 2 55845 3 16000 11169 4800 15969. A mass in grams numerically equal to the molecular weight contains one mole of molecules which is known to be 602 x 1023 Avogadros number. Calculate how many moles are mentioned in the question.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how do you convert molecular weight to grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.