Your How do you change grams to moles images are available in this site. How do you change grams to moles are a topic that is being searched for and liked by netizens today. You can Download the How do you change grams to moles files here. Find and Download all royalty-free vectors.
If you’re searching for how do you change grams to moles pictures information related to the how do you change grams to moles topic, you have visit the right site. Our site always provides you with suggestions for seeing the highest quality video and image content, please kindly hunt and locate more informative video content and graphics that match your interests.
How Do You Change Grams To Moles. If youre asked to supply a number in grams you convert back from the moles used in the calculation. Which of the following reactions are spontaneous favorable. Now lets apply our understanding to calculate the pH of the buffer solution in the following example. The mole is thus the link between the.
 Moley Moley Guacamole Chris Eiffler Formulas Grams To Moles Grams X 1 Mol Of Moles Molar Mass Grams Moles To Grams Moles X Molar Mass Ppt Download From slideplayer.com
Moley Moley Guacamole Chris Eiffler Formulas Grams To Moles Grams X 1 Mol Of Moles Molar Mass Grams Moles To Grams Moles X Molar Mass Ppt Download From slideplayer.com
Lets now apply equation 3 and 4 to solve the following problem. For example to find the amount of NaCl sodium chloride in 200 g one would do the following. We can rearrange this equation in terms of moles n and then solve for its value. The disclaimer is simple. 0184 mole ZnS x 9744 g ZnSmole ZnS 179. Then we divide the number of moles by the total solution volume to get concentration.
Convert grams of the substance given in the problem to moles.
In chemistry courses youll frequently have to convert moles to molecules or molecules to moles using Avogadros number. Now lets apply our understanding to calculate the pH of the buffer solution in the following example. To do this youll want to be familiar with the definitions of both moles and molecules the relationship between the two concepts and the exact formula which uses Avogadros number. Understand the definition of a mole and determine the Avogadro constant by adding atoms or formula units to a balance until the mass in grams is equal to the atomic or formula mass. Then use dimensional analysis to convert between particles moles. Which of the following reactions are spontaneous favorable.
 Source: youtube.com
Source: youtube.com
1 mole 746 g 3 grams 3 746 0040 moles Express this as moles per kilogram solution. Now you have 250 ml of water which is about 250 g of water assuming a density of 1 gml but you also have 3 grams of solute so the total mass of the solution is closer to 253 grams than 250. What would be the molality of the solution. Hope that helped. Understand the definition of a mole and determine the Avogadro constant by adding atoms or formula units to a balance until the mass in grams is equal to the atomic or formula mass.
 Source: youtube.com
Source: youtube.com
Suppose you had 5844 grams of NaCl and you dissolved it in exactly 200 kg of pure water the solvent. 0194 2 0389 mol Mg Keep in mind however that this is moles of Mg. Once you calculate the concentration of the hydronium ionH 3 O you will then move on to take the negative log of the hydronium ion concentration -logH 3 O to get the pH of the buffer. Then we divide the number of moles by the total solution volume to get concentration. MolesMg massMg molar massMg How do you calculate the mass of a product in a reaction.
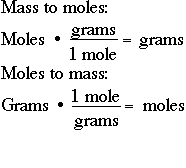 Source: socratic.org
Source: socratic.org
The mole is simply a very large number 6022 xx 1023 that has a special propertyIf I have 6022 xx 1023 hydrogen atoms I have a mass of 1 gram of hydrogen atomsIf I have 6022 xx 1023 H_2 molecules I have a mass of 2 gram of hydrogen molecules. Lets now apply equation 3 and 4 to solve the following problem. Answer 1 of 6. Construct two ratios - one from the problem and one from the chemical equation and set them equal. OR put another way if you blow yourself up dont blame me.
 Source: wikihow.com
Source: wikihow.com
Once the number of moles of a substance present is known we can use. The disclaimer is simple. FindMoles of O grams of glucose moles of glucose moles of oxygen Moles of O 700 g of C 6H 12O x 1mol C H O6 x 6 mol O Molar mass Molar ratio 1801 g mol C6H12 O6 233 mol O Strategy. We do this by dividing by the molecular weight of NaCl 584 gmole. Mass MgO molesMgO molar massMgO a Calculate moles Mg.
 Source: wou.edu
Source: wou.edu
The mole is thus the link between the. What would be the molality of the solution. 10 gm of NaOH is dissolved in 250 ml of water then the molarity of NaOH is calculated by Molarity Weight of NaOH taken 10. Now you have 250 ml of water which is about 250 g of water assuming a density of 1 gml but you also have 3 grams of solute so the total mass of the solution is closer to 253 grams than 250. In chemistry courses youll frequently have to convert moles to molecules or molecules to moles using Avogadros number.
 Source: youtube.com
Source: youtube.com
A 2Mgs O2g - 2MgOs Delta G -1137 kJmol. Molar mass to find the number of grams. Answer 1 of 6. Once the number of moles of a substance present is known we can use. Convert grams of the substance given in the problem to moles.
 Source: slideplayer.com
Source: slideplayer.com
0389 mol4031 gmol 157 g MgO. In chemistry courses youll frequently have to convert moles to molecules or molecules to moles using Avogadros number. How many atoms are in 150 moles of fluorine gas. Answer 1 of 6. Mass MgO molesMgO molar massMgO a Calculate moles Mg.
 Source: study.com
Source: study.com
Comments Double check the equation. To convert to g L-1 you multiply the concentration by the molar mass. 1 mole 746 g 3 grams 3 746 0040 moles Express this as moles per kilogram solution. Which of the following reactions are spontaneous favorable. The disclaimer is simple.
 Source: youtube.com
Source: youtube.com
Comments Double check the equation. After all chemists use balances to weigh things and balances give grams NOT moles. A 2Mgs O2g - 2MgOs Delta G -1137 kJmol. The limiting reactant isnt automatically the one with the smallest number of moles. Convert grams of the substance given in the problem to moles.
 Source: wikihow.com
Source: wikihow.com
How do you convert mol L to G. How many atoms are in 150 moles of fluorine gas. The mole is thus the link between the. Hope that helped. Then use dimensional analysis to convert between particles moles.
 Source: slideplayer.com
Source: slideplayer.com
Determine the mass of the reaction product ZnS by multiplying the number of moles of ZnS produced by the molar mass of ZnS as follows. For example say you have 10 moles of hydrogen and 09 moles of oxygen in the reaction to. FindMoles of O grams of glucose moles of glucose moles of oxygen Moles of O 700 g of C 6H 12O x 1mol C H O6 x 6 mol O Molar mass Molar ratio 1801 g mol C6H12 O6 233 mol O Strategy. Determine the mass of the reaction product ZnS by multiplying the number of moles of ZnS produced by the molar mass of ZnS as follows. For example to find the amount of NaCl sodium chloride in 200 g one would do the following.
 Source: study.com
Source: study.com
Now lets apply our understanding to calculate the pH of the buffer solution in the following example. Manipulate a conceptual model to understand how the number of particles the number of moles and the mass are related. Molar mass to find the number of grams. Now you have 250 ml of water which is about 250 g of water assuming a density of 1 gml but you also have 3 grams of solute so the total mass of the solution is closer to 253 grams than 250. This is much more common.
 Source: uctsc.org
Source: uctsc.org
Determine the mass of the reaction product ZnS by multiplying the number of moles of ZnS produced by the molar mass of ZnS as follows. Understand the definition of a mole and determine the Avogadro constant by adding atoms or formula units to a balance until the mass in grams is equal to the atomic or formula mass. For example to find the amount of NaCl sodium chloride in 200 g one would do the following. Convert grams of the substance given in the problem to moles. Lets now apply equation 3 and 4 to solve the following problem.
 Source: khanacademy.org
Source: khanacademy.org
For example to find the amount of NaCl sodium chloride in 200 g one would do the following. FindMoles of O grams of glucose moles of glucose moles of oxygen Moles of O 700 g of C 6H 12O x 1mol C H O6 x 6 mol O Molar mass Molar ratio 1801 g mol C6H12 O6 233 mol O Strategy. 0389 mol4031 gmol 157 g MgO. Now lets apply our understanding to calculate the pH of the buffer solution in the following example. The limiting reactant isnt automatically the one with the smallest number of moles.
 Source: study.com
Source: study.com
Convert grams of the substance given in the problem to moles. If you are given a value in grams you need to convert it to moles. So we will have to multiply by the molar mass of MgO. Though everything should be awesome I take no responsibility for physical mental moral or metaphysical injuries and the consequences thereof. Which of the following reactions are spontaneous favorable.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how do you change grams to moles by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.