Your How do you calculate the number of moles in a compound images are ready in this website. How do you calculate the number of moles in a compound are a topic that is being searched for and liked by netizens now. You can Find and Download the How do you calculate the number of moles in a compound files here. Find and Download all royalty-free vectors.
If you’re searching for how do you calculate the number of moles in a compound pictures information linked to the how do you calculate the number of moles in a compound topic, you have visit the right site. Our site always gives you hints for refferencing the highest quality video and picture content, please kindly surf and find more informative video articles and images that match your interests.
How Do You Calculate The Number Of Moles In A Compound. The answer is the number of moles of that mass of compound. Moles of element n moles elementone mole of compound K -. Calculate the number of moles of gas from the given pressure volume and temperature. As you can see from the screenshot above Nickzom Calculator The Calculator Encyclopedia solves for the molar mass and presents the formula workings and steps too.
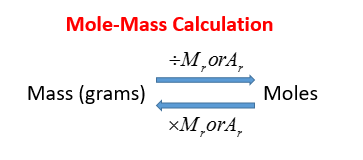 Mole Calculation Video Lessons Examples And Solutions From onlinemathlearning.com
Mole Calculation Video Lessons Examples And Solutions From onlinemathlearning.com
Number of moles 95 8694. The answer is the number of moles of that mass of compound. Ask me questions on Facebook. Determine the moles of product produced by dividing the grams of product by the grams per mole of product. Ions have oxidation numbers equal to their charge. Lets start with these values.
How to Find Moles.
Besides for calculating molarity we use the following equation. 9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion. Calculate the number of moles of gas from the given pressure volume and temperature. Lets start with these values. Best hikes near crystal mountain. Therefore there are 500 g 1011 gmol 00495 mol of KNO3.
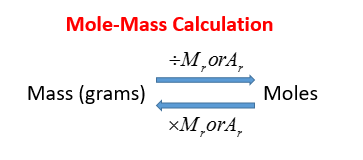 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Molarity moles of solute litres of solution. 1811022 x 5 there are 5 atoms in NH4 9061022 Nmber of NH4 ions 181 x 10 22 ions and the number of moles of NH4 ions 00301 moles Fourth convert that to mols. Determine the moles of product produced by dividing the grams of product by the grams per mole of product. First of all before you can use this equation you need to know how many moles of solute are there in the solution. Number of moles mass relative formula mass This can be rearranged to find the mass if the number of moles and molar mass its relative formula mass in grams are known.
 Source: thefactfactor.com
Source: thefactfactor.com
Divide each mole value by the smallest number of moles calculated. Besides for calculating molarity we use the following equation. The answer is the number of moles of that mass of compound. This is thoroughly answered here. Solved Examples On Number Of Moles Formulas.
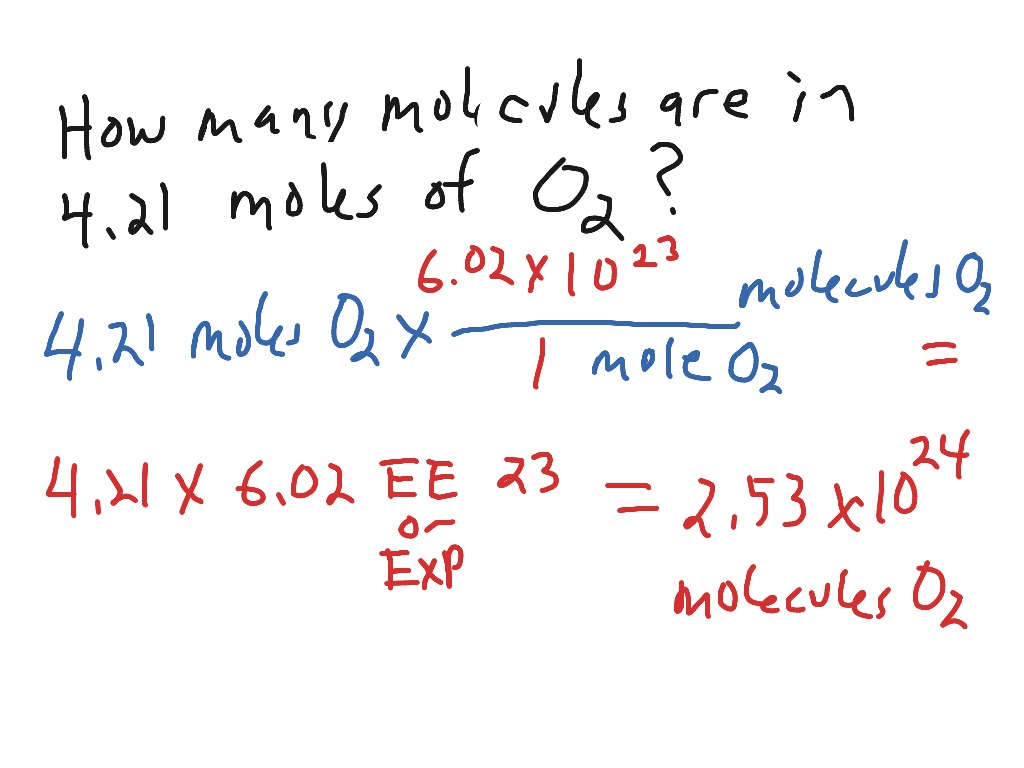 Source: showme.com
Source: showme.com
You now have calculated the number of moles of every compound used in this reaction. Specifically 1 mole represents 6022 x 1023 atoms or molecules of substance. Mass of one mole MnO 2 8694g. In other words it tells you the number of. Peach smoothie bowl without yogurt.

Divide each mole value by the smallest number of moles calculated. Specifically 1 mole represents 6022 x 1023 atoms or molecules of substance. Hence number of moles in calcium is 2. Calculate the number of moles of gas from the given pressure volume and temperature. Ions have oxidation numbers equal to their charge.
 Source: pharmafactz.com
Source: pharmafactz.com
How to calculate number of moles. How to Find Moles. 100 g CaCl 2. N the number of moles of a compound. Mass of MnO 2 95g.
 Source: youtube.com
Source: youtube.com
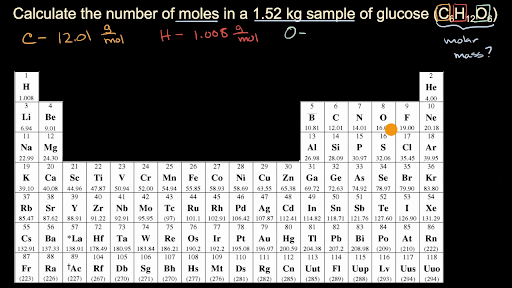
So for every one mole of glucose C6H12O6 we have 18016 grams of glucose C6H12O6 and this is going to get us we get 152 times 1000 is equal to this is the number of grams of glucose we have and then were going to divide by 18016 divide by 18016 gives us this number and lets see if we see significant figures we have three significant figures here we have five here so. How do you find how many grams are in a mole. Determine the moles of product produced by dividing the grams of product by the grams per mole of product. Answer 1 of 3. Moreover we express it as M.
 Source: dummies.com
Source: dummies.com
This is the mole ratio of the elements and is. Moreover we express it as M. 200 mL H 2 O. The mole represents a quantity of substance but relates to the number of atoms or molecules rather than mass or volume. N the number of moles of a compound.
 Source: youtube.com
Source: youtube.com
Besides for calculating molarity we use the following equation. 1811022 x 5 there are 5 atoms in NH4 9061022 Nmber of NH4 ions 181 x 10 22 ions and the number of moles of NH4 ions 00301 moles Fourth convert that to mols. N the number of moles of a compound. Mass of MnO 2 95g. Calculate the number of moles of gas from the given pressure volume and temperature.
To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compound. Number of moles formula is. This is the mole ratio of the elements and is. Specifically 1 mole represents 6022 x 1023 atoms or molecules of substance. Suppose that you have 500 g of KNO3.
 Source: youtube.com
Source: youtube.com
Round to the nearest whole number. Mass of MnO 2 95g. First of all before you can use this equation you need to know how many moles of solute are there in the solution. Specifically 1 mole represents 6022 x 1023 atoms or molecules of substance. 200 mL H 2 O.
 Source: slideplayer.com
Source: slideplayer.com
Mass of MnO 2 95g. For example 25 grams of water equals 2518016 or 139 moles. Round to the nearest whole number. You now have calculated the number of moles of every compound used in this reaction. Suppose that you have 500 g of KNO3.
 Source: wikihow.com
Source: wikihow.com
Ions have oxidation numbers equal to their charge. Calculate the number of moles of gas from the given pressure volume and temperature. Answer 1 of 3. The mole represents a quantity of substance but relates to the number of atoms or molecules rather than mass or volume. Hence number of moles in calcium is 2.
 Source: surfguppy.com
Source: surfguppy.com
Therefore there are 500 g 1011 gmol 00495 mol of KNO3. For example 25 grams of water equals 2518016 or 139 moles. Moles of element n moles elementone mole of compound K -. Divide the number of grams of each reactant by the number of grams per mole for that reactant. 21749 moles of na are used in this reaction.
 Source: youtube.com
Source: youtube.com
9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion. Moles of element n moles elementone mole of compound K -. Calculate the number of moles of gas from the given pressure volume and temperature. How to Calculate Number of Moles. Number of moles Mass of substance Mass of one mole.
 Source: khanacademy.org
Source: khanacademy.org
Divide the mass of the compound in grams by the molar mass you just calculated. Number of moles Mass of substance Mass of one mole. 100 g CaCl 2. Number of moles 95 8694. Round to the nearest whole number.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how do you calculate the number of moles in a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.