Your How do u find grams per mole images are ready in this website. How do u find grams per mole are a topic that is being searched for and liked by netizens today. You can Download the How do u find grams per mole files here. Get all royalty-free photos and vectors.
If you’re looking for how do u find grams per mole pictures information linked to the how do u find grams per mole topic, you have come to the ideal site. Our website frequently provides you with hints for viewing the highest quality video and image content, please kindly surf and find more informative video articles and graphics that fit your interests.
How Do U Find Grams Per Mole. Since the unified atomic mass unit symbol. Now you can use these numbers in the equations. One mole contains exactly 6022 140 76 10 23 elementary entities. For example a molecule has a molecular weight of 18018 gmol.
 How To Convert Grams To Moles Video Lesson Transcript Study Com From study.com
How To Convert Grams To Moles Video Lesson Transcript Study Com From study.com
Thus the number of moles in the given sample of. For H 2. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. However balances give readings in grams. Now you can use these numbers in the equations. Energy given out mass of water 42 temperature change.
Thus the number of moles in the given sample of.
So the problem is that in chemistry we compare amounts of one substance to another using moles. So the problem is that in chemistry we compare amounts of one substance to another using moles. Besides one mole of Sodium Chloride NaCl has a mass of approximately 585 grams. Do coefficients affect molar mass. Since the unified atomic mass unit symbol. Energy measured in joules J.
 Source: pinterest.com
Source: pinterest.com
So the problem is that in chemistry we compare amounts of one substance to another using moles. In chemistry the mole is the standard measurement of amount. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. Determine the mass in grams of each element in the sample. More free chemistry help videos.
 Source: study.com
Source: study.com
And it gives the conversion factor of 1 585. 159994 x 2 319988 grams per mole. You can also enter any custom value for molar mass. 35453 x 2 70096 grams per mole. Determine the mass in grams of each element in the sample.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
In our example the weight of NaCl is 100 grams and its molecular weight is 5852 gmoles. When finding the molar mass of the reactants you use the number of moles given in the problem and multiply it by the molar mass of that element or compound to get its mass. Find the chemical of your desire from the second list. You can also enter any custom value for molar mass. For H 2.
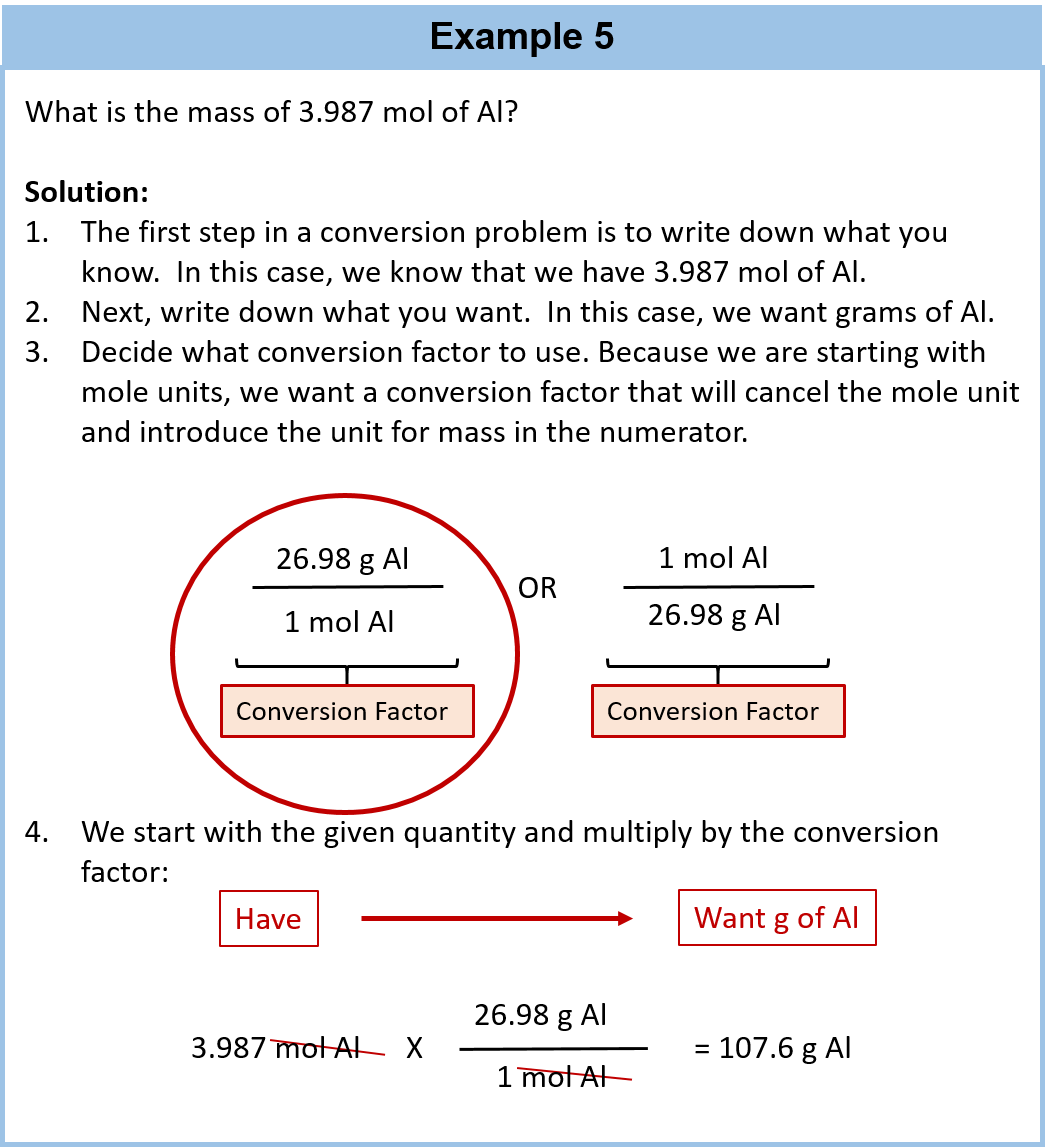 Source: wou.edu
Source: wou.edu
Determine the mass in grams of each element in the sample. When substances react they do so in simple ratios of moles. What do we multiply moleslitre by to get gramslitre. The units of molar mass are grams per mole abbreviated as gmol. So the problem is that in chemistry we compare amounts of one substance to another using moles.
 Source: slideplayer.com
Source: slideplayer.com
35453 x 2 70096 grams per mole. This tells us that there are 0427 moles of NaCl in the solution. 159994 x 2 319988 grams per mole. Now we can use the rearranged equation. Find the chemical of your desire from the second list.
 Source: study.com
Source: study.com
1 grams per mole 0001 grams per millimole using the online calculator for metric conversions. So the problem is that in chemistry we compare amounts of one substance to another using moles. Now multiply 25 g with 1 585 which is same as dividing 25 585. Now we are already in litres so how to convert moles to grams. More free chemistry help videos.
 Source: slideplayer.com
Source: slideplayer.com
159994 x 2 319988 grams per mole. When substances react they do so in simple ratios of moles. 35453 x 2 70096 grams per mole. So the molar enthalpy change H 84 001136 739 kJmol. Number of moles Weight of compound in grams molecular weight of compound.
 Source: khanacademy.org
Source: khanacademy.org
Total molar mass 19988 gramsmole. Find the chemical of your desire from the second list. 159994 x 2 319988 grams per mole. Total molar mass 19988 gramsmole. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da.
 Source: youtube.com
Source: youtube.com
You can also enter any custom value for molar mass. We use molar mass which is grams per mole. So the molar enthalpy change H 84 001136 739 kJmol. However balances give readings in grams. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da.
 Source: study.com
Source: study.com
For example a molecule has a molecular weight of 18018 gmol. Moles mol x Molar Mass gmol 1 x 5844. Now use the number of moles and multiply it by the molar mass. You start by determining the empirical formula for the compound. This tells us that there are 0427 moles of NaCl in the solution.
 Source: slideplayer.com
Source: slideplayer.com
Do a quick conversion. For H 2. Besides one mole of Sodium Chloride NaCl has a mass of approximately 585 grams. Moles mol Molarity M x Volume L 05 x 2. One mole contains exactly 6022 140 76 10 23 elementary entities.
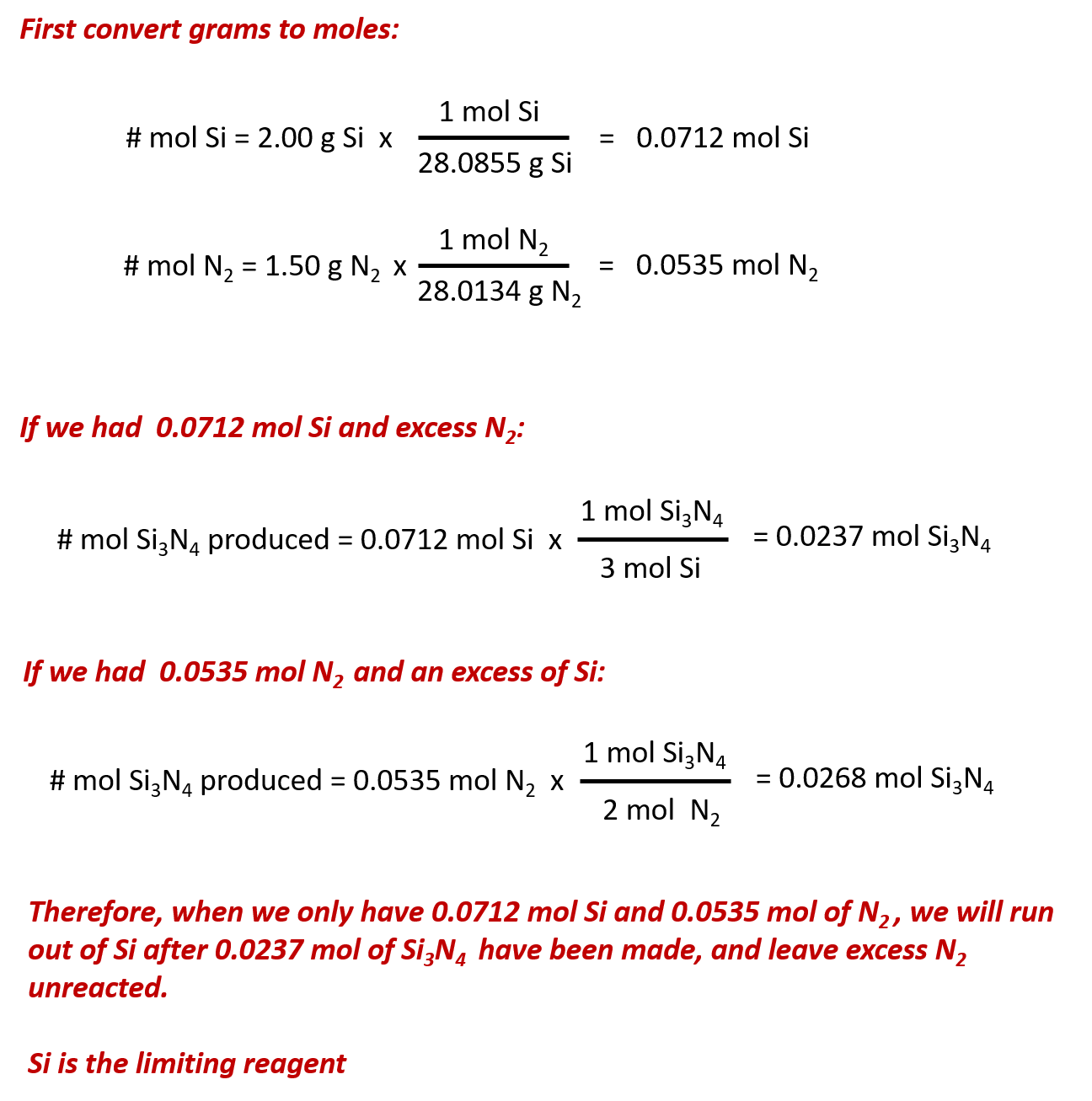 Source: wou.edu
Source: wou.edu
Two nitrogen 14012 2802 gramsmole. And for Cl 2. We use molar mass which is grams per mole. So the problem is that in chemistry we compare amounts of one substance to another using moles. More free chemistry help videos.
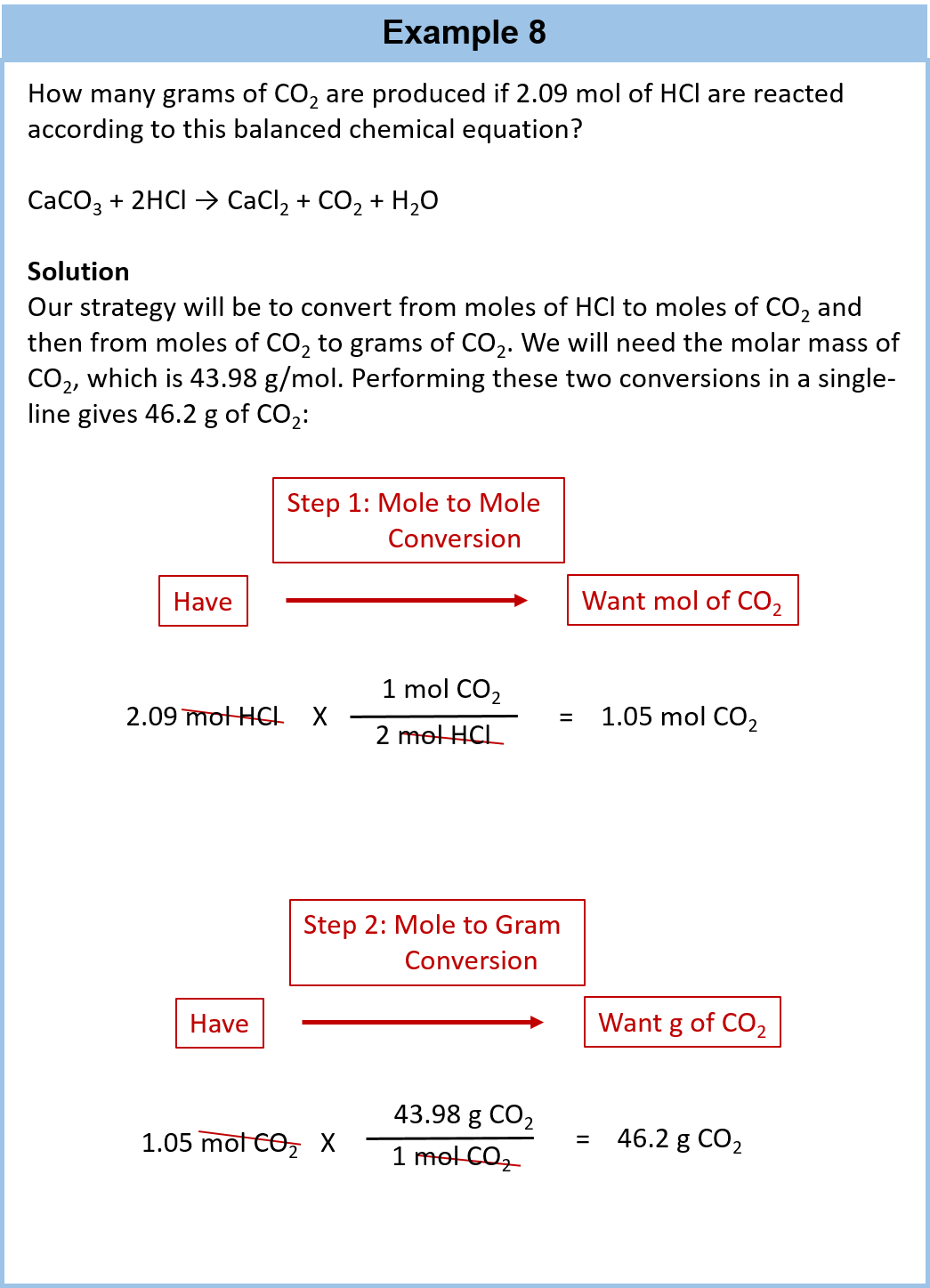 Source: wou.edu
Source: wou.edu
Since the unified atomic mass unit symbol. Now multiply 25 g with 1 585 which is same as dividing 25 585. Now you can use these numbers in the equations. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula. First you must calculate the number of moles in this solution by rearranging the equation.
 Source: youtube.com
Source: youtube.com
Total molar mass 19988 gramsmole. 35453 x 2 70096 grams per mole. Now we are already in litres so how to convert moles to grams. One calcium 4008 gramsmole. Find the chemical of your desire from the second list.
 Source: slideplayer.com
Source: slideplayer.com
And it gives the conversion factor of 1 585. Now multiply 25 g with 1 585 which is same as dividing 25 585. Besides one mole of Sodium Chloride NaCl has a mass of approximately 585 grams. Check the chart for more details. Total molar mass 19988 gramsmole.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how do u find grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






