Your How do u convert from grams to moles images are available in this site. How do u convert from grams to moles are a topic that is being searched for and liked by netizens now. You can Download the How do u convert from grams to moles files here. Download all royalty-free photos.
If you’re looking for how do u convert from grams to moles pictures information related to the how do u convert from grams to moles keyword, you have come to the ideal site. Our website always gives you suggestions for downloading the maximum quality video and picture content, please kindly surf and locate more enlightening video content and images that fit your interests.
How Do U Convert From Grams To Moles. How to go from moles to grams. This is often associated with atoms andor molecules but you can apply it to others too although it. 1 mole is equal to 1 moles O2 or 319988 grams. 1 mole is equal to 1 moles U or 23802891 grams.
 How To Convert Between Moles Atoms And Grams In Chemistry Quick Simple Youtube From youtube.com
How To Convert Between Moles Atoms And Grams In Chemistry Quick Simple Youtube From youtube.com
How many moles are in 7537 grams of sodium chloride NaCl. Grams Moles x Molar Mass. A sample of 12 grams of carbon is equal to one moleThe amount of moles in a substance can be determined using that substances molar mass. Moles to Grams Conversion FormulaIn order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. 1 mole is equal to 1 moles U or 23802891 grams. Convert 250 g of water to moles of water.
How many moles are in a gram.
First find the molar mass of NaCl. First find the molar mass of NaCl. We know that 1 mol H₂O 1802 g H₂O. How do you calculate moles from grams. This is often associated with atoms andor molecules but you can apply it to others too although it. 1 gmol 0001 kgmol Measurement calculator that can be used to convert gmol to kgmol among others.
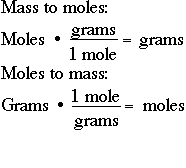 Source: socratic.org
Source: socratic.org
1 mole is equal to 1 moles O2 or 319988 grams. How many moles are in a gram. How do you convert grams to molecules. We assume you are converting between moles In and gram. A sample of 12 grams of carbon is equal to one moleThe amount of moles in a substance can be determined using that substances molar mass.
 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles U or 23802891 grams. This comes out be 5555 moles. Formula to convert moles to grams. Molecular weight of U or grams The SI base unit for amount of substance is the mole. 1 gmol 0001 kgmol Measurement calculator that can be used to convert gmol to kgmol among others.
 Source: wikihow.com
Source: wikihow.com
The SI base unit for amount of substance is the mole. How many moles are in a gram. Following step three we obtain. Answer 1 of 2. There are exactly 31773 grams in the given quantity of copper that could also be calculated by using a free online moles to grams calculator.
 Source: xaktly.com
Source: xaktly.com
We can use this to create the conversion factors 1 mol H_2O1802 g H_2O and 1802 g H_2O1 mol H_2O. So to convert from grams to moles divide the amount in grams by the atomic weight in grams to find the amount of moles. How do you calculate moles from grams. Moles to Grams Conversion FormulaIn order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. You create a conversion factor from the molar mass and use it in your calculations.
 Source: youtube.com
Source: youtube.com
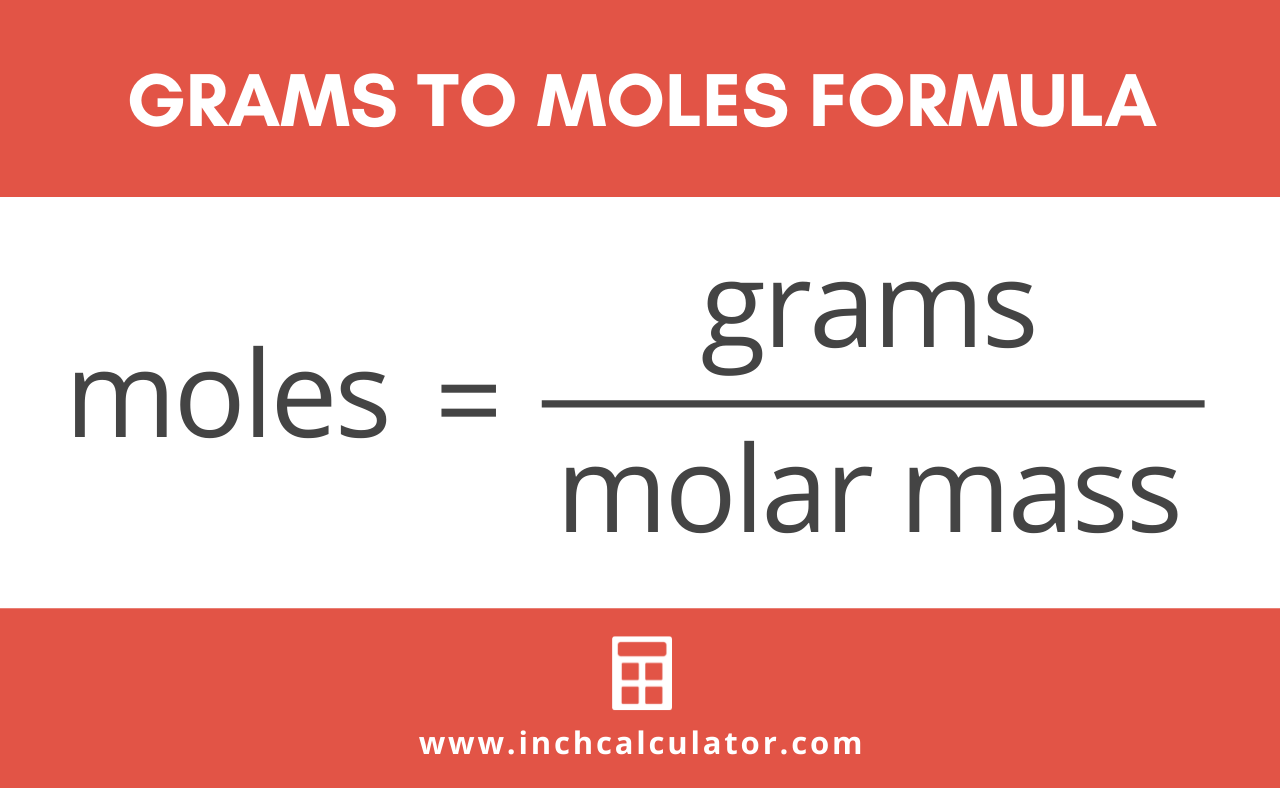
So in order to calculate the number of moles of any substance present in the sample we simply divide. The molar mass is the amount of grams in one mole of a substance. More commonly written for this application as. There are exactly 31773 grams in the given quantity of copper that could also be calculated by using a free online moles to grams calculator. Divide the number of grams of the substance by the molecular mass.
 Source: inchcalculator.com
Source: inchcalculator.com
Formula to convert moles to grams. Amazingly there are 602x1023 atoms in each of the samples above. Grams to Moles Conversion Formula Questions. The molar mass is the amount of grams in one mole of a substance. Following step three we obtain.
 Source: khanacademy.org
Source: khanacademy.org
Grams Moles x Molar Mass. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. First of all find the number of moles by using mass and molar mass. You can view more details on each measurement unit. 1 mole is equal to 1 moles O2 or 319988 grams.
 Source: study.com
Source: study.com
250 moles x 122550 gmole 306375 grams. Convert 250 g of water to moles of water. First of all find the number of moles by using mass and molar mass. You can convert the grams to molecules by following the steps below. 1 mole is equal to 1 moles U or 23802891 grams.
 Source: study.com
Source: study.com
Divide the number of grams of the substance by the molecular mass. How to go from moles to grams. How do you calculate moles from grams. How do you calculate moles from molar mass. Its the number of particles contained in one mole of substance.
 Source: youtube.com
Source: youtube.com
The grams cancel because they are both on the bottom of the equation if you make the amount in grams a fraction over 1 gram which leaves moles and the answer. 1 mole is equal to 1 moles NH3 or 1703052 grams. Finally divide the number of grams of the compound by the molar mass of the compound to find the number of moles. You can convert the grams to molecules by following the steps below. Divide the number of grams of the substance by the molecular mass.
 Source: wikihow.com
Source: wikihow.com
250 moles x 122550 gmole 306375 grams. More commonly written for this application as. Formula to convert moles to grams. You can view more details on each measurement unit. First of all find the number of moles by using mass and molar mass.
 Source: youtube.com
Source: youtube.com
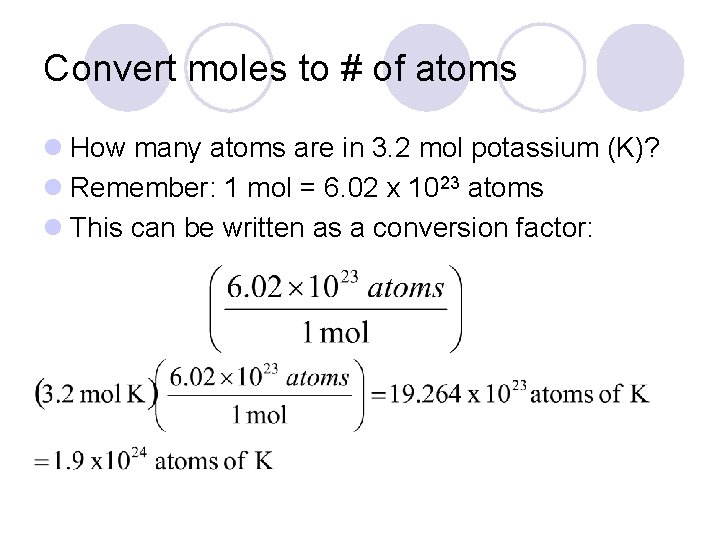
This is equal to the number of grams in one mole of the substance. So in order to calculate the number of moles of any substance present in the sample we simply divide. How do you calculate moles from grams. Divide the number of grams of the substance by the molecular mass. How many moles of electrons are in 1 kg.
 Source: study.com
Source: study.com
Then add all of your answers together to find the molar mass of the compound. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions. 250 moles x 122550 gmole 306375 grams. Molecular weight of In or grams The SI base unit for amount of substance is the mole. Consequently how do you convert mass to moles.
 Source: slidetodoc.com
Source: slidetodoc.com
How do you calculate moles from molar mass. Molecular weight of In or grams The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles In or 114818 grams. 02 x 5844 11688 grams. 1 mole is equal to 1 moles NH3 or 1703052 grams.
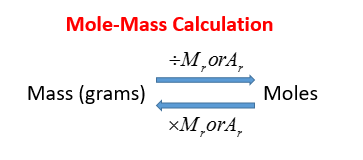 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Formula to convert moles to grams. To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compound. Following step three we obtain. One mole of oxygen atoms contains 6022141791023 oxygen atoms. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how do u convert from grams to moles by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






