Your How do i find grams per mole images are ready. How do i find grams per mole are a topic that is being searched for and liked by netizens today. You can Find and Download the How do i find grams per mole files here. Download all royalty-free vectors.
If you’re searching for how do i find grams per mole pictures information related to the how do i find grams per mole topic, you have visit the right blog. Our website always gives you suggestions for refferencing the highest quality video and image content, please kindly hunt and find more enlightening video content and graphics that fit your interests.
How Do I Find Grams Per Mole. Shows how to use molar conversions to convert from grams to moles and moles to grams. 21008 3206 418 106076. In 4788 grams of titanium there is one mole or 6022 x 10 23 titanium atoms. And it gives the conversion factor of 1 585.
 Calculating Moles And Grams Of Solute From Molality Youtube From youtube.com
Calculating Moles And Grams Of Solute From Molality Youtube From youtube.com
First divide by 1000 to convert to gramsin this case 01 grams. Using the periodic table of the elements to find atomic weights we find that hydrogen has an atomic weight of 1 and oxygens is 16. 1007 x 2 2014 grams per mole. 250 moles x 122550 gmole 306375 grams. The tool is really user friendly and pretty straightforward to use. 3 6 1 0 3 moles of h 2 to produce ammonia.
Shows how to use molar conversions to convert from grams to moles and moles to grams.
For H 2. Now multiply 25 g. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula. In 4788 grams of titanium there is one mole or 6022 x 10 23 titanium atoms. To convert this to kJgram you need to now divide by the atomic weight of aluminum 2698 gmmol. And for Cl 2.
 Source: clutchprep.com
Source: clutchprep.com
For O2 thisis approximately 319988. 867 grams 90 grams per mole 096 moles 8. This can be converted to grams by multiplying the number of moles by the molar mass of the solute. Since water has two molecules of hydrogen and one molecule of oxygen then the molecular weight of water is 1801528gmol. Sample Molecular Weight Calculation.

3 Following step three we obtain. 159994 x 2 319988 grams per mole. 3 Following step three we obtain. To go from grams to moles divide the grams by the molar mass. 1007 x 2 2014 grams per mole.
 Source: wikihow.com
Source: wikihow.com
159994 x 2 319988 grams per mole. For O2 thisis approximately 319988. In order to calculate the molecular weight of one water molecule we add the contributions from each atom. The tool is really user friendly and pretty straightforward to use. 50 stoichiometry worksheet answer key en 2020.
 Source: youtube.com
Source: youtube.com
Usually the units used for this are grams per moleIn this movie we show how to calculate the molecular weight of a substance from the atomic weights given on the periodic table. 250 moles x 122550 gmole 306375 grams. Have No Fear Of Ice Cold Beverages Hydrogen Nitrogen Fluorine Oxygen Iodine Chlorine Bromine. For a molecule with a weight of 18 the molar mass is 18 grams. Therefore for 16 grams of water there is 089 moles.
 Source: youtube.com
Source: youtube.com
In order to calculate the molecular weight of one water molecule we add the contributions from each atom. Shows how to use molar conversions to convert from grams to moles and moles to grams. For H 2. Since there are 2 moles of aluminum you need to divide this ΔH by 2 then you have heat released in kJmol of aluminum. 250 moles x 122550 gmole 306375 grams.
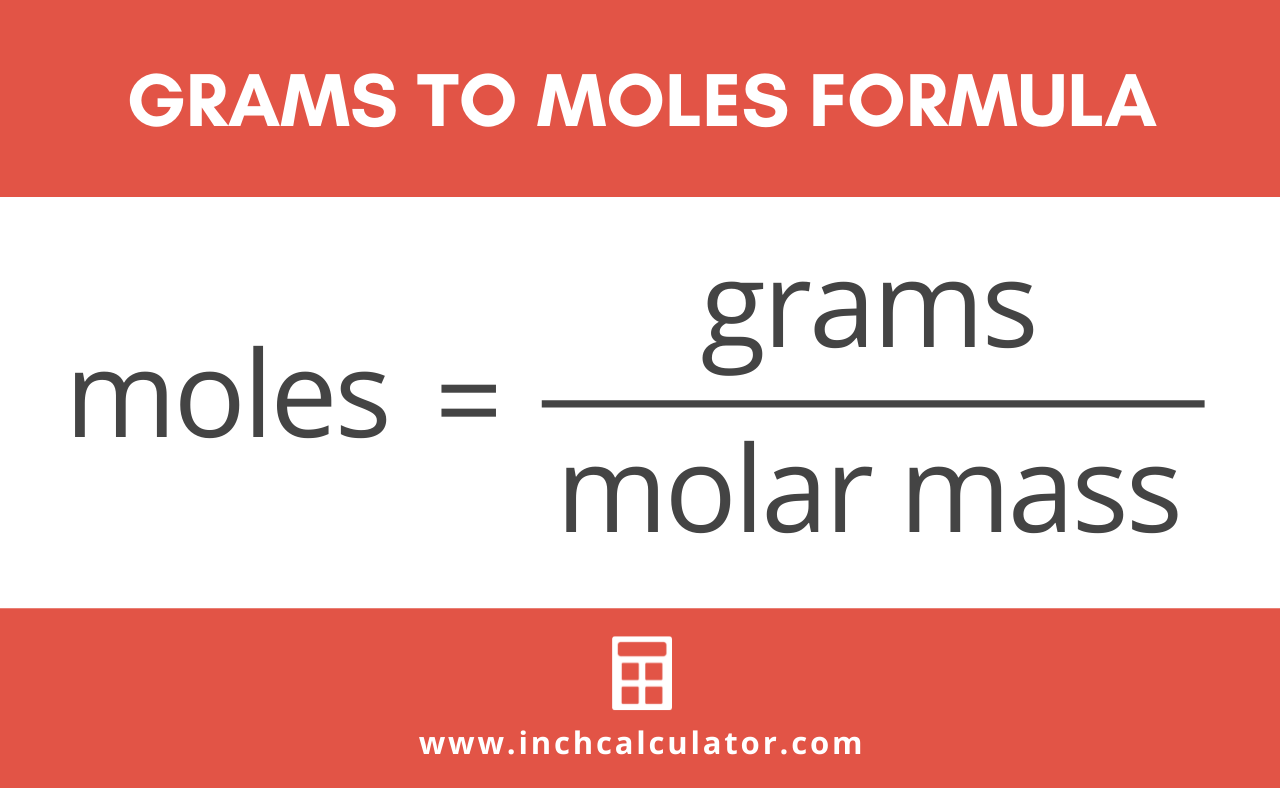 Source: inchcalculator.com
Source: inchcalculator.com
Now multiply 25 g. Calculating Molecular Weight Molar MassThe molecular weight is the mass of one mole of a substance. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula. Shows how to use molar conversions to convert from grams to moles and moles to grams. Hence one mole of H 2 SO 4 weights 106076 grams.
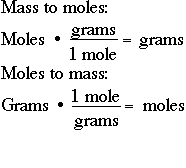 Source: socratic.org
Source: socratic.org
Since water has two molecules of hydrogen and one molecule of oxygen then the molecular weight of water is 1801528gmol. And for Cl 2. For example the atomic mass of titanium is 4788 amu or 4788 gmol. 3 6 1 0 3 moles of h 2 to produce ammonia. If you know the volume of the solution in liters you can multiply the molarity by the volume in liters to determine the number of moles of solute.
 Source: youtube.com
Source: youtube.com
50 stoichiometry worksheet answer key en 2020. It is important for proper cancelling of units that you remember to write this unit down when using a molar mass. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula. One mnemonic device for remembering diatomic elements molecules of 2 atoms is. The answer should be rounded off to three significant figures resulting in 306 g.
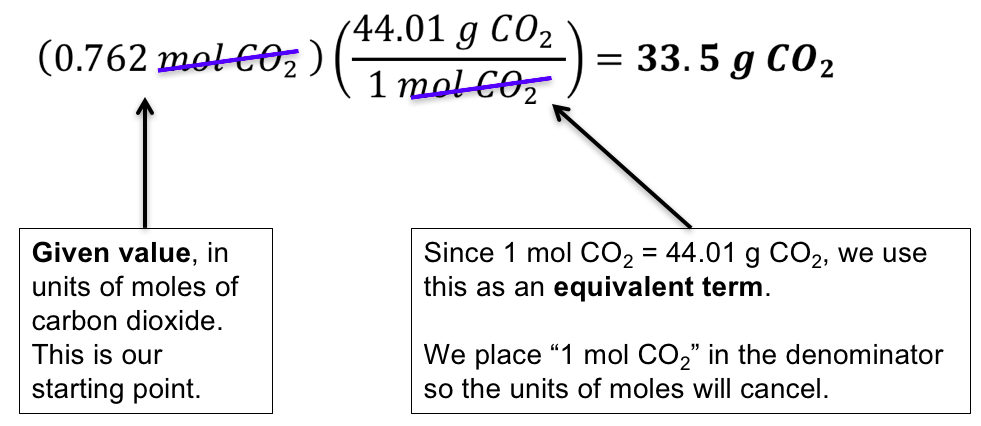 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
The answer should be rounded off to three significant figures resulting in 306 g. Besides one mole of Sodium Chloride NaCl has a mass of approximately 585 grams. For example if you have 170 mol of NaCl then. And for Cl 2. The formula for moles to grams is given by.
 Source: khanacademy.org
Source: khanacademy.org
Now multiply 25 g. For example the atomic mass of titanium is 4788 amu or 4788 gmol. 250 moles x 122550 gmole 306375 grams. The molar mass is the mass of one mole of a substance. You can also enter any custom value for molar mass.
 Source: wikihow.com
Source: wikihow.com
The molar mass is the mass of one mole of a substance. And for Cl 2. To go from moles to grams multiply by the formula mass. To convert this to kJgram you need to now divide by the atomic weight of aluminum 2698 gmmol. Molarity is the concentration of a solution in units of moles of solute per liter of solution.
 Source: study.com
Source: study.com
170 mol 58443 g 1 mol 994 g. 867 grams 90 grams per mole 096 moles 8. And for Cl 2. Molarity is the concentration of a solution in units of moles of solute per liter of solution. Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol.
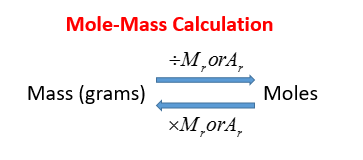 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Therefore the molecular mass of H 2 SO 4 is. 35453 x 2 70096 grams per mole. How many grams are in 379 moles of calcium bromide CaBr 2. The answer should be rounded off to three significant figures resulting in 306 g. How To Find Limiting Reagent With Moles.
 Source: khanacademy.org
Source: khanacademy.org
One mnemonic device for remembering diatomic elements molecules of 2 atoms is. In order to calculate the molecular weight of one water molecule we add the contributions from each atom. Hence one mole of H 2 SO 4 weights 106076 grams. 600 g 58443 gmol 1027 mol of NaCl. Now use the number of moles and multiply it by the molar mass.
 Source: wikihow.com
Source: wikihow.com
3 6 1 0 3 moles of h 2 to produce ammonia. 3 6 1 0 3 moles of h 2 to produce ammonia. The answer should be rounded off to three significant figures resulting in 306 g. 159994 x 2 319988 grams per mole. Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how do i find grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.