Your How do i convert moles into grams images are available. How do i convert moles into grams are a topic that is being searched for and liked by netizens now. You can Download the How do i convert moles into grams files here. Find and Download all free images.
If you’re searching for how do i convert moles into grams pictures information linked to the how do i convert moles into grams keyword, you have come to the right blog. Our website always provides you with suggestions for downloading the maximum quality video and picture content, please kindly search and find more informative video content and graphics that match your interests.
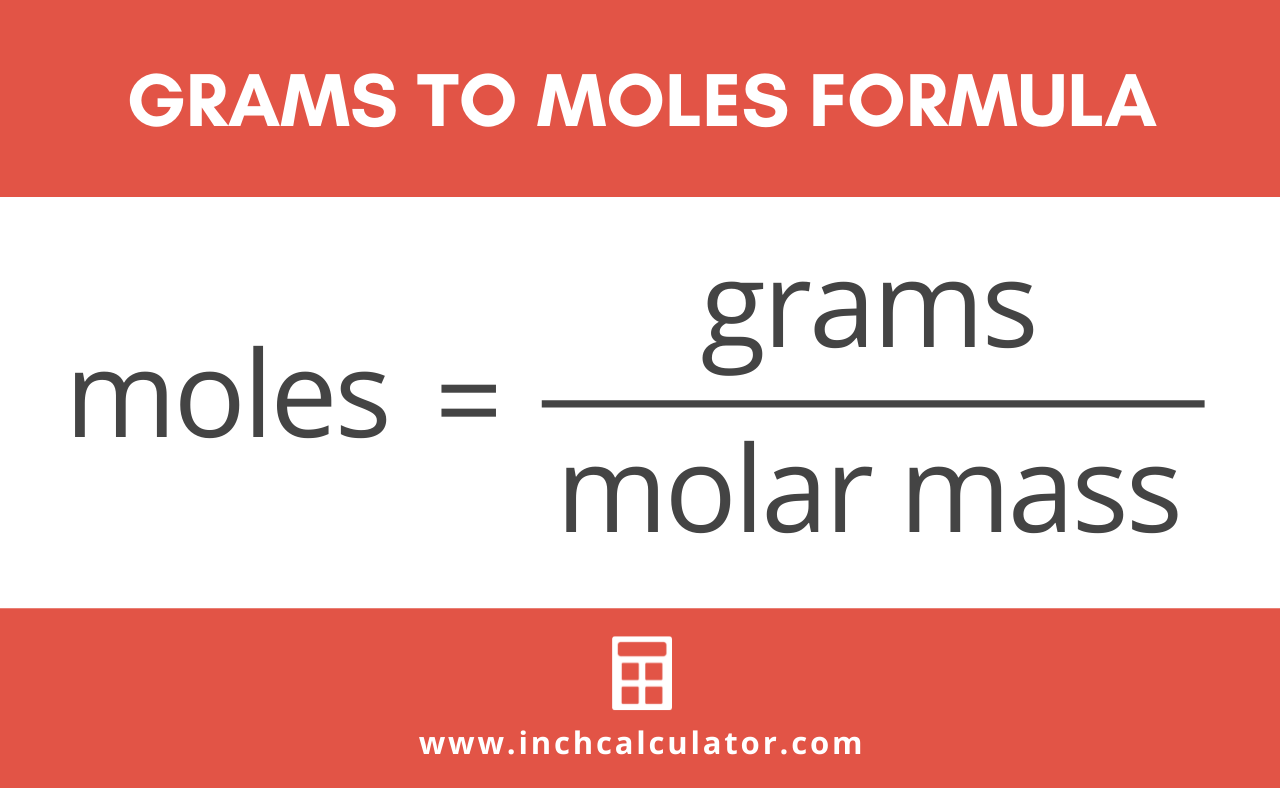
How Do I Convert Moles Into Grams. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. Two moles have a mass of 218 grams 36 grams. The mole is the SI unit of the measurement for the amount of a substance.
 Hubert Hudson Fold Frog Convert Moles To Grams Calculator Uctsc Org From uctsc.org
Hubert Hudson Fold Frog Convert Moles To Grams Calculator Uctsc Org From uctsc.org
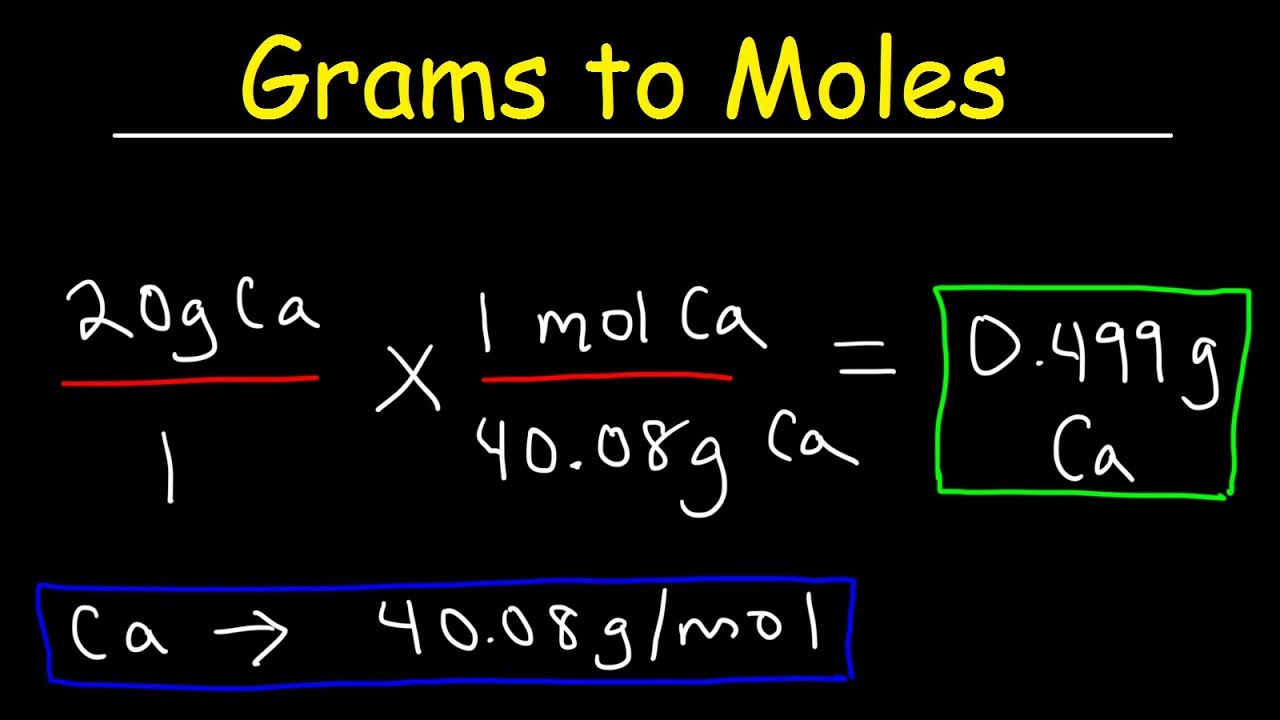
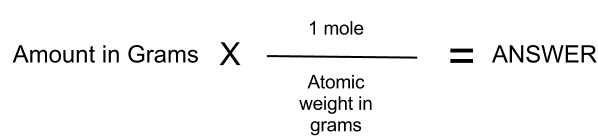
How to Convert Grams to Moles. For example imagine you have 2 g of water or H 2 O and you want to convert it to molesThe molecular mass of H 2 O is 18gmol. Two moles have a mass of 218 grams 36 grams. Formula to convert moles to grams. N m M where M is the molar mass of this material. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams.
Check the chart for more details.
Where is the molar mass of the substance. You can convert molarity into grams per liter by multiplying it with the molecular Molar mass ie. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. Grams Moles x Molar Mass. Using a calculator divide the number of grams by the molar mass. As the correct answer.
 Source: youtube.com
Source: youtube.com
The mole is the SI unit of the measurement for the amount of a substance. 250 moles x 122550 gmole 306375 grams The answer should be rounded off to three significant figures resulting in 306 g. Find the molar mass of the substance. Convert 139 mol of water to grams of water. One mole consists of Avogadro number of atoms.
 Source: uctsc.org
Source: uctsc.org
Check the chart for more details. 4 moles in to grams 459272 grams. N m M where M is the molar mass of this material. One mole consists of Avogadro number of atoms. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass.
 Source: youtube.com
Source: youtube.com
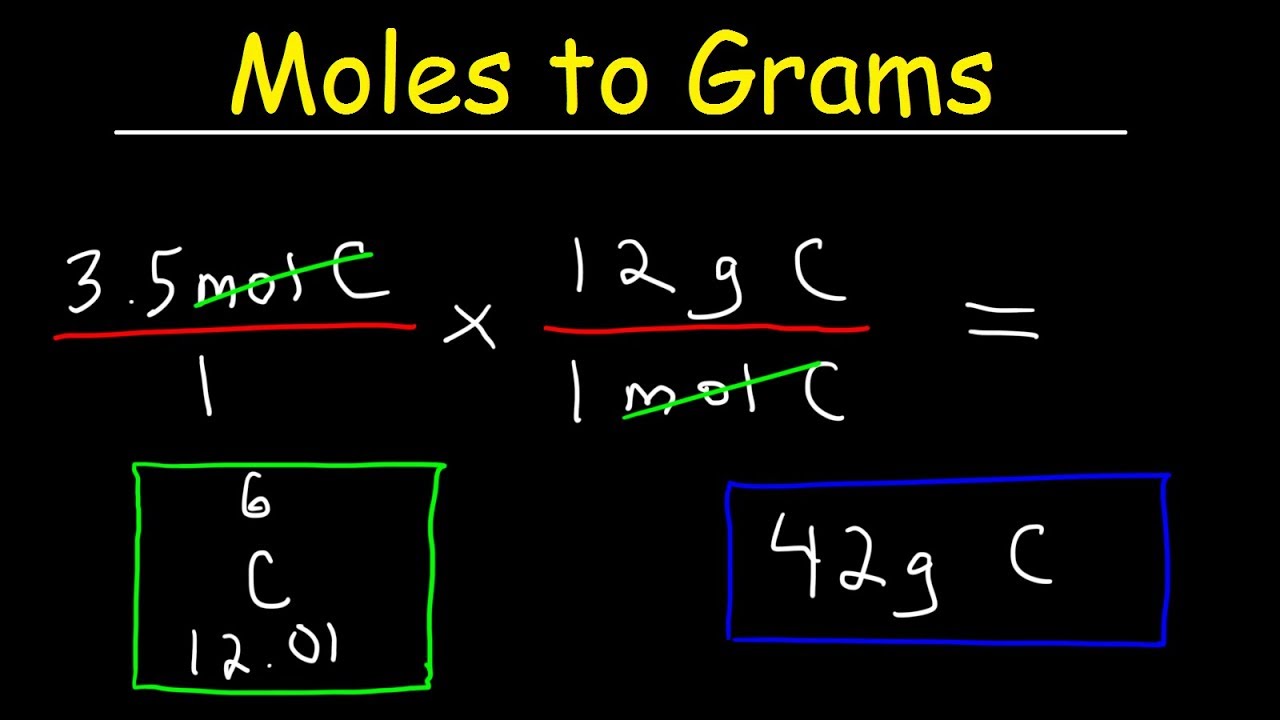
The result is the number of moles in your element or compound. Find the molar mass of the substance. What is Meant by Moles to Grams. To convert the moles into grams multiply the mass of the substance by the molecular weight formula weight. Converting grams to moles involves 2 steps.
 Source: youtube.com
Source: youtube.com
The number of gramsMol. Grams to moles conversion formula. You can convert molarity into grams per liter by multiplying it with the molecular Molar mass ie. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. 1 moles In 114818 gram using the molecular weight calculator and the molar mass of In.
 Source: uctsc.org
Source: uctsc.org
Convert 02 moles of Sodium chloride. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. Check the chart for more details. In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight. Where is the molar mass of the substance.
 Source: uctsc.org
Source: uctsc.org
Multiply the number of moles by the molar mass to obtain the final answer in grams. The molecular weight of water is 18 amu so one mole of water has a mass of 18 grams. You can convert molarity into grams per liter by multiplying it with the molecular Molar mass ie. In other words it is the product of the mass. Grams to moles conversion formula.
 Source: khanacademy.org
Source: khanacademy.org
I n Chemistry the moles to grams conversion represents the conversion of moles into grams. 1 moles 116 116 gram using the molecular weight calculator and the molar mass of 116. Find the molecular mass of the compound. Calculate how many moles are mentioned in the question. 1 moles In 114818 gram using the molecular weight calculator and the molar mass of In.
 Source: uctsc.org
Source: uctsc.org
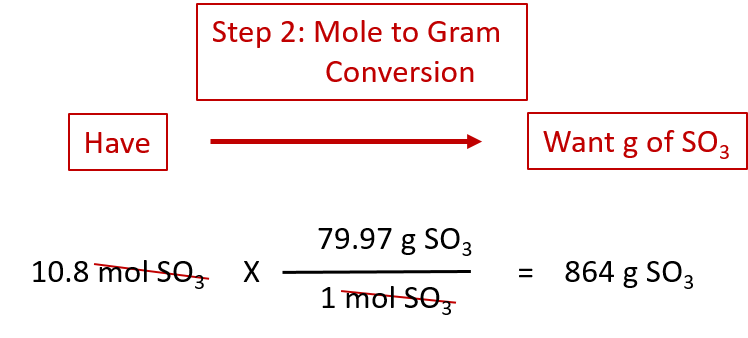
In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight. Multiply the number of moles by the molar mass to obtain the final answer in grams. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. Grams Moles x Molar Mass. N m M where M is the molar mass of this material.
 Source: wou.edu
Source: wou.edu
In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight. So we choose the first one and write 250 g H₂O 1 mol H_2O1802 g H_2O 139 mol H₂O Example 2. More commonly written for this application as. This dimensional analysis video tuto. The molecular weight of water is 18 amu so one mole of water has a mass of 18 grams.
 Source: uctsc.org
Source: uctsc.org
So we choose the first one and write 250 g H₂O 1 mol H_2O1802 g H_2O 139 mol H₂O Example 2. The unit is typically gmol. So if you are given a number of moles multiply it by the number corresponding to the atomic. The mole is the SI unit of the measurement for the amount of a substance. Multiply the number of moles by the molar mass to obtain the final answer in grams.
 Source: wikihow.com
Source: wikihow.com
Convert 139 mol of water to grams of water. Multiply both the values. 1 moles 116 116 gram using the molecular weight calculator and the molar mass of 116. To convert moles into grams determine the number of moles preset and the molar mass of the compound. Find the molar mass of the substance.
 Source: hu.pinterest.com
Source: hu.pinterest.com
Converting grams to moles involves 2 steps. This is equal to the number of grams in one mole of the substance. N m M where M is the molar mass of this material. The mole is the SI unit of the measurement for the amount of a substance. So if you are given a number of moles multiply it by the number corresponding to the atomic.
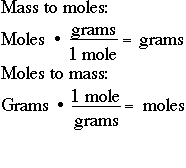 Source: socratic.org
Source: socratic.org
02 x 5844 11688 grams. Multiply the number of moles by the molar mass to obtain the final answer in grams. As the correct answer. These measurements are used to determine the number of substances. More commonly written for this application as.
 Source: abetterchemtext.com
Source: abetterchemtext.com
The result is the number of moles in your element or compound. One mole consists of Avogadro number of atoms. Do a quick conversion. Determine the number of moles. Do a quick conversion.
 Source: study.com
Source: study.com
Grams Moles x Molar Mass. Find the molar mass of the substance. Two moles have a mass of 218 grams 36 grams. Divide the number of grams of the compound by its molecular mass. In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how do i convert moles into grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.