Your How can you find the mole ratio of a compound images are available in this site. How can you find the mole ratio of a compound are a topic that is being searched for and liked by netizens now. You can Get the How can you find the mole ratio of a compound files here. Get all free photos and vectors.
If you’re searching for how can you find the mole ratio of a compound images information related to the how can you find the mole ratio of a compound topic, you have come to the ideal site. Our site frequently provides you with suggestions for viewing the maximum quality video and image content, please kindly search and locate more informative video articles and graphics that fit your interests.
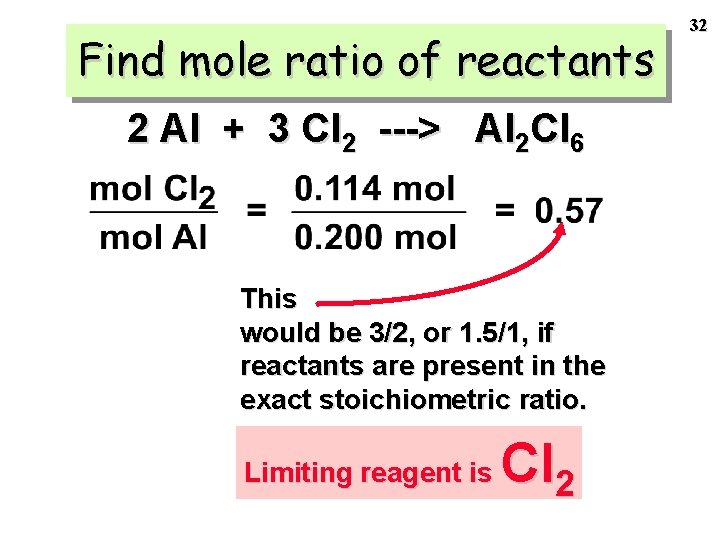
How Can You Find The Mole Ratio Of A Compound. 354 g of Cr X 1 mole Cr 520 g Cr 0681 moles Cr. Choose a signal and calculates the area of H separately of each compound. You will actually be determining the mole ratios of the products through mass analysis. Mole ratios are the central step in performing stoichiometry because they allow us to convert moles of one substance to moles of another.
 Mole Review 1 Calculate The Number Of Moles From slidetodoc.com
Mole Review 1 Calculate The Number Of Moles From slidetodoc.com
Number of moles of Y333 666333 2. For each compound find the grams of copper that combine with 100 g of chlorine by dividing the mass of copper by the mass of chlorine. How do you find the ratio of a molecule. Let us say you are asked Calculate how many moles MgCl2 are in a sample that contains 486 g Mg. Molar mass Mg 243 gmol You have determined that the. What is the ratio of an element.
The molecular formula of a compound can be found with the use of empirical formula.
For each compound find the grams of copper that combine with 100 g of chlorine by dividing the mass of copper by the mass of chlorine. Divide the Mass of Each Element Present by the Molar Mass. Hydrate —— anhydrous salt water. There are four steps in solving a stoichiometry problem. Convert the units of the given substance A to moles. The molar ratio can be constructed using any two compounds in the reaction be they reactants or products.
 Source: slidetodoc.com
Source: slidetodoc.com
Determining Molar Ratios The molar ratios identify how many moles of product are formed from a certain amount of reactant as well as the number of moles of a reactant needed to completely react with a certain amount of another reactant. Number of moles massformula mass. Number of moles of X333 333333 1. 265 g of K X 1 mole K 391 g K 0678 moles K. 354 g of Cr X 1 mole Cr 520 g Cr 0681 moles Cr.
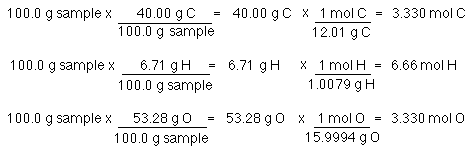 Source: westfield.ma.edu
Source: westfield.ma.edu
Convert the units of the given substance A to moles. You will actually be determining the mole ratios of the products through mass analysis. The empirical formula is the simplest whole-number ratio of atoms in a compound. Determine the Mass of Each Individual Element Present. Elemental Ratios A ratio is a way to compare quantities of things.
 Source: slidetodoc.com
Source: slidetodoc.com
Here the smallest value is 333 moles. Elemental Ratios A ratio is a way to compare quantities of things. The ratio between them is the molar ratio. It does not give the exact number of each atom present. The empirical formula is the simplest whole-number ratio of atoms in a compound.
 Source: youtube.com
Source: youtube.com
The molecular formula of a compound can be found with the use of empirical formula. Assume you have 100g of this stuff so it would be. Here the smallest value is 333 moles. Empirical formula expresses the simplest mole ratio of the elements in a compound or molecule. Sometimes a color change occurs when a colored.
 Source: youtube.com
Source: youtube.com
0493 g 0297 g mass of O. Mass of magnesium oxide mass of Mg mass of O. Determining a Mole Ratio. 265 g of K X 1 mole K 391 g K 0678 moles K. Number of moles of Y333 666333 2.
 Source: chem.libretexts.org
Source: chem.libretexts.org
You do this conversion by assuming that you have 100 g of your compoundKeep in mind that this 10000 g is just a definition. Then find the ratio of the masses of copper in the two compounds by dividing the larger value. Answer 1 of 2. Hydrate —— anhydrous salt water. The molecular formula of a compound can be found with the use of empirical formula.
 Source: slideplayer.com
Source: slideplayer.com
These values can be used to calculate the molar mass of a compound by multiplying the quantity of each element by its molar mass. The symbol represents heat. Mass of Mg 0297 g. 0493 g 0297 g mass of O. Here the smallest value is 333 moles.
 Source: nagwa.com
Source: nagwa.com
It does not give the exact number of each atom present. The empirical formula is the chemical formula which gives the ratio between the atoms present in the compound. Hydrate Analysis Lab. The molecular formula of a compound can be found with the use of empirical formula. Salt and can be removed upon heating yielding the anhydrous salt salt without water.
 Source: bulbapp.com
Source: bulbapp.com
You have a compound made of 265 K 354 Cr and 381 O. The molecular formula of a compound can be found with the use of empirical formula. For each compound find the grams of copper that combine with 100 g of chlorine by dividing the mass of copper by the mass of chlorine. 354 g of Cr X 1 mole Cr 520 g Cr 0681 moles Cr. Convert moles of the wanted substance to the desired units.
 Source: slideplayer.com
Source: slideplayer.com
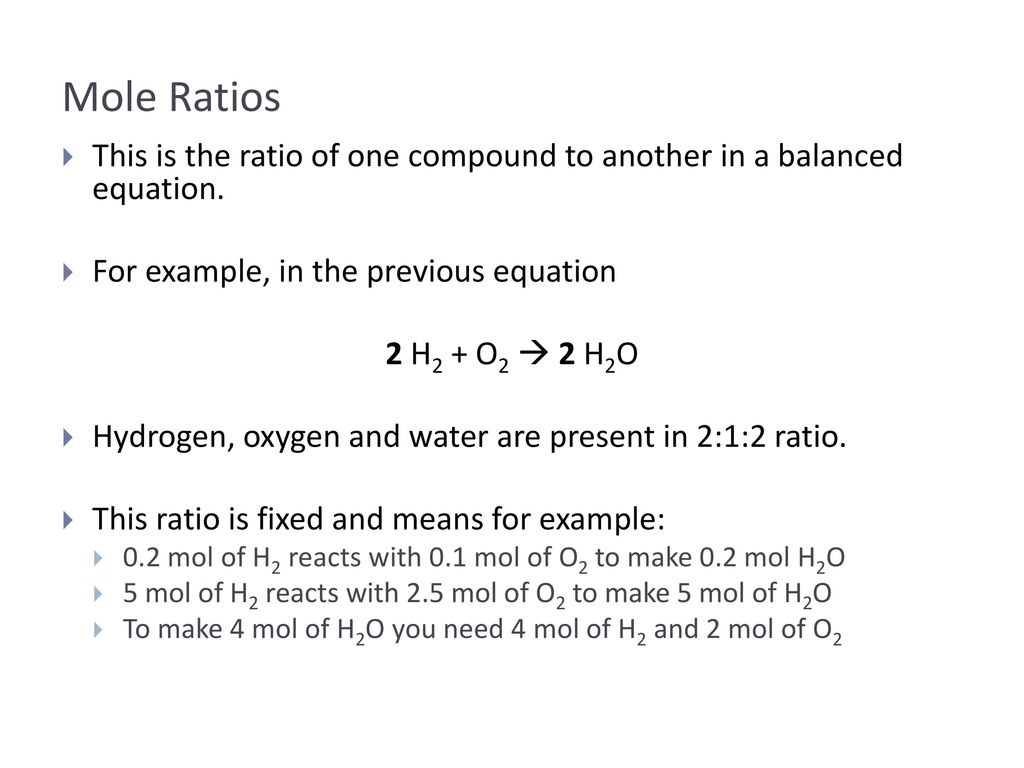
For each compound find the grams of copper that combine with 100 g of chlorine by dividing the mass of copper by the mass of chlorine. The molar ratio from the balanced equation must be considered to tell us how many moles of aluminium will. Also to know is what is a mole reaction. Also to do this you need to know the volume of the solution and how many solutes has been dissolved in the solution. Elemental Ratios A ratio is a way to compare quantities of things.
 Source: pinterest.com
Source: pinterest.com
The molar ratio from the balanced equation must be considered to tell us how many moles of aluminium will. Assume you have 100g of this stuff so it would be. These values can be used to calculate the molar mass of a compound by multiplying the quantity of each element by its molar mass. How to find moles in the solution is to calculate how many molecules the solution contains. Number of moles of X333 333333 1.
 Source: dummies.com
Source: dummies.com
First you have to know what compound you are dealing with. Convert mole ratios into whole numbers. So for example the chemical formula for water is H 20 which means there are two moles of hydrogen for every the subscript on oxygen is one although not written for every one mole of oxygen. How do you find the ratio of a molecule. The molecular formula of a compound can be found with the use of empirical formula.
 Source: slideplayer.com
Source: slideplayer.com
You do this conversion by assuming that you have 100 g of your compoundKeep in mind that this 10000 g is just a definition. Convert moles of the wanted substance to the desired units. The ratio of moles is 1 to 1 so the empirical formula would be PbS. How to Calculate Mass Ratio. Number of moles of X333 333333 1.
 Source: quizlet.com
Source: quizlet.com
The empirical formula is the chemical formula which gives the ratio between the atoms present in the compound. Mass of magnesium oxide mass of Mg mass of O. The fractional numbers of atoms composing the chemical formula were found by knowing the molecular mass and respecting the indicated mole ratio CuFe of 133100. Number of moles of Y333 666333 2. What is the ratio of an element.
 Source: youtube.com
Source: youtube.com
There are four steps in solving a stoichiometry problem. Can be calculated from the ratio of moles of anhydrous salt to moles of water. First you have to know what compound you are dealing with. Molar mass Mg 243 gmol You have determined that the. Number of moles massformula mass.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how can you find the mole ratio of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






