Your Grams in one mole of glucose images are available. Grams in one mole of glucose are a topic that is being searched for and liked by netizens today. You can Get the Grams in one mole of glucose files here. Download all royalty-free photos and vectors.
If you’re looking for grams in one mole of glucose images information connected with to the grams in one mole of glucose topic, you have visit the right site. Our website frequently gives you suggestions for downloading the maximum quality video and picture content, please kindly search and locate more enlightening video content and images that fit your interests.
Grams In One Mole Of Glucose. Mass of 602210 23 molecule 180ℊ. Thus 1 mole of glucose has 180 ℊ of mass. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon. Note that I am labouring the point that we deal with OXYGEN ATOMS NOT OXYGEN MOLECULES.

1 moles Glucose 18015588 gram utilizing the molecular weight calculator and the molar mass of C6H12O6. There are 51171021 molecules present in 153g of C6H12O6 glucose. So turning back to the question. Glucose C_6H_12O_6 has a molecular mass of 18016gmol-1. Mr of glucose is 6x12 12x1 6x16 180 gmol because Mr. 1 mole is equal to 1 moles Glucose or 18015588 grams.
Use this page to learn how to convert between grams Glucose and mole.
N mMr. Glucose C_6H_12O_6 has a molecular mass of 18016gmol-1. So number of moles of glucose in 900 g 900180 5. 1 mole is equal to 1 moles Glucose or 18015588 grams. Molar mass of C 12 g mol-1. Use this page to learn how to convert between grams Glucose and mole.
 Source: sciencetrends.com
Source: sciencetrends.com
The SI base unit for amount of substance is the mole. You can view more details on each measurement unit. 180 g of glucose 1 mole of glucose. From the chemical structure of glucose acetate one mole is 306 grams the molar mass or molecular weight is 306 gmole. Use this page to learn how to.
 Source: clutchprep.com
Source: clutchprep.com
So number of moles of glucose in 900 g 900180 5. Note that rounding errors may occur so always check the results. Molar mass of C 12 g mol-1. Because CLEARLY each mole of glucose C6H 12O6 contains 6 mol oxygen atoms. From the calculation we know that when one mole or 180 grams of glucose burns it releases 4950 kJ of energy the heat of reaction is -4950 kJmole.
 Source: youtube.com
Source: youtube.com
Ask me if you have any doubts. Molecular weight of Glucose or grams The molecular formula for Glucose is C6H12O6. Hence from molecular formula itself we can calculate that its molar mass is 180 gmole. Melting Point MP Glucose changes its state from solid to liquid at 146C 2948F. Thus 1 mole of glucose has 180 ℊ of mass.
 Source: youtube.com
Source: youtube.com
Note that I am labouring the point that we deal with OXYGEN ATOMS NOT OXYGEN MOLECULES. Do a quick conversion. 1 mole is equal to 1 moles Glucose or 18015588 grams. 1 atom of 126𝐶 weighs 12000 amu u 6 1 Whats the mass of 1 atom of carbon. From the chemical structure of glucose acetate one mole is 306 grams the molar mass or molecular weight is 306 gmole.
 Source: khanacademy.org
Source: khanacademy.org
How many grams are there in 2 moles of glucose. Therefore Mass of 1 molecule 602210 23180. The molar mass of glucose can be calculated from the molar masses of individual atoms present in it. There are 00085 or 85103 moles present. Calculate the amount of carbon dioxide evolved if 440 grams of propane C 3 H 8 is combusted with oxygenAlso calculate the number of moles of.

Type in your own numbers in the form to convert the units. One mole is 6021023 molecules. So number of moles of glucose in 900 g 900180 5. Use this page to learn how to convert between grams Glucose and mole. From the molecular formula C 6 H 12 O 6 one can find there are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in one molecule of glucose.
 Source: youtube.com
Source: youtube.com
Note that rounding errors may occur so always check the results. The SI base unit for amount of substance is the mole. Use this page to learn how to convert between grams Glucose and mole. Molar mass of H 1 g mol-1. Density of glucose is equal to 1 560 kgm³In Imperial or US customary measurement system the density is equal to 97388 pound per cubic foot lbft³ or 09017 ounce per cubic inch ozinch³.
 Source: toppr.com
Source: toppr.com
1 grams Glucose is equal to 00055507486072617 mole. One mole of glucose molecule has a mass of 18016 g. Therefore Mass of 1 molecule 602210 23180. From the chemical structure of glucose one mole is 180 grams the molar mass or molecular weight is 180 gmole. This equation describes the relationship between the number of moles n the mass m and the relative molecular mass Mr.

Mass of 602210 23 molecule 180ℊ. There are 51171021 molecules present in 153g of C6H12O6 glucose. Glucose is an organic molecule or particularly a reducing hexaaldose whose molecular formula is C6H12O6. 1 mole is equal to 1 moles Glucose or 18015588 grams. So molar mass of glucose 612011 121008 615999 180156 Hence 20 grams of glucose is 0111 moles of glucose Avogadros number 60221023 There are 6 moles of carbon in each mole of glucose.
 Source: clutchprep.com
Source: clutchprep.com
From the calculation we know that when one mole or 180 grams of glucose burns it releases 4950 kJ of energy the heat of reaction is -4950 kJmole. Density of glucose is equal to 1 560 kgm³In Imperial or US customary measurement system the density is equal to 97388 pound per cubic foot lbft³ or 09017 ounce per cubic inch ozinch³. A mole is the quantity of a substance whose weight in grams is equal to the molecular weight of the substance. Calculate the amount of carbon dioxide evolved if 440 grams of propane C 3 H 8 is combusted with oxygenAlso calculate the number of moles of. Thus 1 mole of glucose weighs 180 g.
 Source: clutchprep.com
Source: clutchprep.com
There are 00085 or 85103 moles present. You can view more details on each measurement unit. There are 00085 or 85103 moles present. From the calculation we know that when one mole or 180 grams of glucose burns it releases 2560 kJ of energy the heat of reaction is -2560 kJmole. 1 grams Glucose is equal to 00055507486072617 mole.
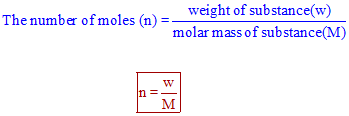 Source: adichemistry.com
Source: adichemistry.com
1 mole is equal to 1 moles Glucose or 18015588 grams. Hence from molecular formula itself we can calculate that its molar mass is 180 gmole. Do a quick conversion. 180 g of glucose 1 mole of glucose. Density of glucose is equal to 1 560 kgm³In Imperial or US customary measurement system the density is equal to 97388 pound per cubic foot lbft³ or 09017 ounce per cubic inch ozinch³.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
The SI base unit for amount of substance is the mole. 96 g oxygen is a molar quantity of 96 g 16. The SI base unit for amount of substance is the mole. The SI base unit for amount of substance is the mole. Use this page to learn how to.
 Source: slideplayer.com
Source: slideplayer.com
There are 51171021 molecules present in 153g of C6H12O6 glucose. Because CLEARLY each mole of glucose C6H 12O6 contains 6 mol oxygen atoms. The molar mass of glucose can be calculated from the molar masses of individual atoms present in it. There are 00085 or 85103 moles present. Type in your own numbers in the form to convert the units.
 Source: youtube.com
Source: youtube.com
1 atom of 126𝐶 weighs 12000 amu u 6 1 Whats the mass of 1 atom of carbon. So there will probably be 900180 5 moles of glucose in 900 grams of glucose. Calculate the amount of carbon dioxide evolved if 440 grams of propane C 3 H 8 is combusted with oxygenAlso calculate the number of moles of. There are 51171021 molecules present in 153g of C6H12O6 glucose. The SI base unit for amount of substance is the mole.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title grams in one mole of glucose by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






