Your Finding number of moles of an element in a compound images are available. Finding number of moles of an element in a compound are a topic that is being searched for and liked by netizens now. You can Get the Finding number of moles of an element in a compound files here. Download all free vectors.
If you’re looking for finding number of moles of an element in a compound pictures information connected with to the finding number of moles of an element in a compound topic, you have pay a visit to the ideal site. Our site frequently provides you with suggestions for refferencing the maximum quality video and picture content, please kindly hunt and locate more informative video articles and images that match your interests.
Finding Number Of Moles Of An Element In A Compound. Suppose that you have 500 g of KNO3. K - 00495 mol. If you have 1000 grams. Mar 1 2019 Simply divide the mass with units of grams by the molar mass with units of grams PER mole And so if got a mass of we divide thru by the molar mass.
 Calculating The Moles Of An Element In A Compound Youtube From youtube.com
Calculating The Moles Of An Element In A Compound Youtube From youtube.com
1811022 x 5 there are 5 atoms in NH4 9061022 Nmber of NH4 ions 181 x 1022 ions and the number of moles of NH4 ions 00301 moles. How do you find how many grams are in a mole. We assume you are converting between moles H2O and gram. NaCl Na Cl. So if you are given the mass of an element you use the periodic table to find its molar mass and multiply the given mass by the reciprocal of the molar mass. After trying myself I derived the equation A p m a where A is the number of atoms p is the percent composition m is the.
As shown in this video we can obtain a substances molar mass by summing the molar masses of its component atoms.
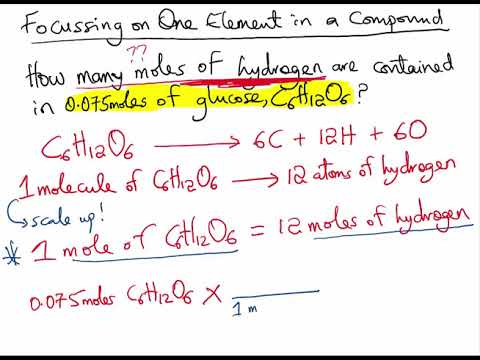
Moles elementone mole of compound Okay 1 mole of Okay for 1 mole of KNO3. 04052018 Calculate the variety of O atoms. How to calculate number of moles of specific element in a co. 1811022 x 5 there are 5 atoms in NH4 9061022 Nmber of NH4 ions 181 x 1022 ions and the number of moles of NH4 ions 00301 moles. Then 1000 g 151001 gmol. This quantity is called Avogadros number.
 Source: youtube.com
Source: youtube.com
The unit is denoted by mol. How many atoms of each element are found in. N - 00495 mol. This quantity is called Avogadros number. So nNNa – n Na N number of atoms 2 1grmol H 2 moles H x 5 moles in the compound 10 moles.
 Source: youtube.com
Source: youtube.com
NaCl Na Cl. NaCl Na Cl. It can be eq6times 10 23 eq atoms of. For example one molecule of H2O has two atoms of Hydrogen H. O - 3 moles of O for 1 mole of KNO3.
 Source: pinterest.com
Source: pinterest.com
O 300495 mol 0148 mol. Answer 1 of 3. Now in two moles of H2 12044 1023 molecules are present and in 1 molecule of H2 two H atoms are present. It can be used to measure the products obtained from the chemical reaction. How to calculate number of moles of specific element in a co.
 Source: youtube.com
Source: youtube.com
Lets say I am given a certain mass of a compound. It can be used to measure the products obtained from the chemical reaction. N - 00495 mol. Example If we are told the mass of each element present in a compound we can find the formula. 9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion.
 Source: khanacademy.org
Source: khanacademy.org
Calculate the formula mass or molar mass of any given compound. We assume you are converting between moles H2O and gram. Popular Answers 1 Calculate he number of moles you have by taking the Mass molar mass. Moles O 545 g O x 1 mol O 1600 g O 341 mol O. O - 3 moles of O for 1 mole of KNO3.
 Source: youtube.com
Source: youtube.com
Although the stoichiometric coefficients could be fractions entire numbers are often used and sometimes most popular. N the number of moles of a compound. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. 04052018 Calculate the variety of O atoms. After trying myself I derived the equation A p m a where A is the number of atoms p is the percent composition m is the.
 Source: pinterest.com
Source: pinterest.com
Therefore NaCl 584538 gL. Then add all of your answers together to find the molar mass of the compound. This video explains how to calculate the number of moles of an element given the mass as well as how to calculate the mass given the number of moles. To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compound. One mole of any substance is equal to the value of 6023 x 10 23 Avagadro number.
 Source: pinterest.com
Source: pinterest.com
Simply so how do you find the number of moles of an element in a compound. Then 1000 g 151001 gmol. Calculate the formula mass or molar mass of any given compound. If you have 1000 grams. For locating out this you need to multiply the mass of solute by.
 Source: pinterest.com
Source: pinterest.com
One mole of any substance is equal to the value of 6023 x 10 23 Avagadro number. 9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. O 3 moles of O for 1 mole of KNO3. So if you are given the mass of an element you use the periodic table to find its molar mass and multiply the given mass by the reciprocal of the molar mass.
 Source: pinterest.com
Source: pinterest.com
You can view more details on each measurement unit. Moles H 458 g H x 1 mol H 101 g H 453 mol H. Determining Number of Moles of a Compound With Known Mass Once youve found the molecular weight you know the weight of one mole of a compound. Jeremiah Daniels on How To Find Moles Of An Element In A Compound Given Mass. N 1 mole of N for 1 mole of KNO3.
 Source: pinterest.com
Source: pinterest.com
How do you find how many grams are in a mole. 1 mole is equal to 1 moles H2O or 1801528 grams. Then 1000 g 151001 gmol. NaCl 229898 gL 354530 gL. 04052018 Calculate the variety of O atoms.
 Source: pinterest.com
Source: pinterest.com
This is Moles Atoms. Then add all of your answers together to find the molar mass of the compound. We assume you are converting between moles H2O and gram. O 3 moles of O for 1 mole of KNO3. Moles elementone mole of compound Okay 1 mole of Okay for 1 mole of KNO3.
 Source: pinterest.com
Source: pinterest.com
So nNNa – n Na N number of atoms 2 1grmol H 2 moles H x 5 moles in the compound 10 moles. Therefore NaCl 584538 gL. So The number of moles of each element. Moles elementone mole of compound Okay 1 mole of Okay for 1 mole of KNO3. If you have 1000 grams.
 Source: pinterest.com
Source: pinterest.com
04052018 Calculate the variety of O atoms. Moles H 458 g H x 1 mol H 101 g H 453 mol H. O - 300495 mol 0148 mol. Calculate the formula mass or molar mass of any given compound. Moles of element n moles elementone mole of compound K - 1 mole of K for 1 mole of KNO3.
 Source: pinterest.com
Source: pinterest.com
So The number of moles of each element. 1811022 x 5 there are 5 atoms in NH4 9061022 Nmber of NH4 ions 181 x 1022 ions and the number of moles of NH4 ions 00301 moles. O 300495 mol 0148 mol. Now the only confusing thing for me is the 5 moles. Molarity Calculator How do you calculate the number of moles.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title finding number of moles of an element in a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.