Your Does amu equal grams per mole images are ready in this website. Does amu equal grams per mole are a topic that is being searched for and liked by netizens now. You can Find and Download the Does amu equal grams per mole files here. Get all royalty-free images.
If you’re looking for does amu equal grams per mole pictures information connected with to the does amu equal grams per mole interest, you have come to the right blog. Our site frequently provides you with suggestions for seeing the maximum quality video and picture content, please kindly search and locate more informative video content and graphics that fit your interests.
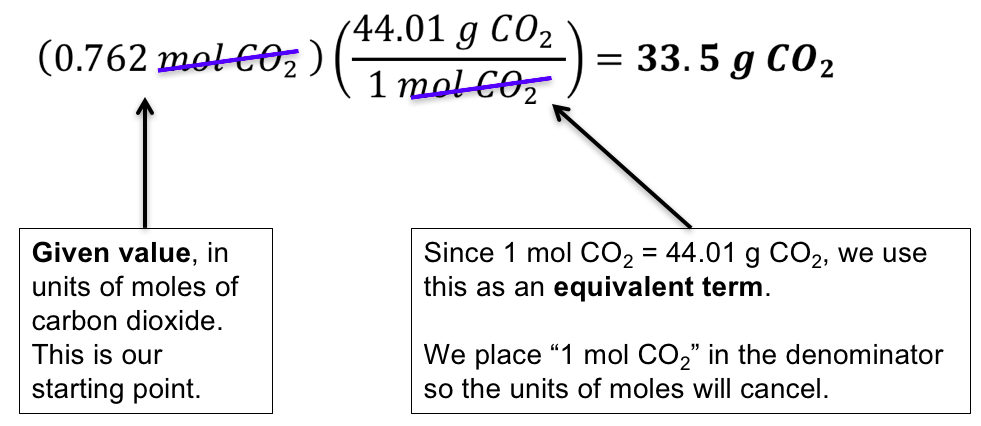
Does Amu Equal Grams Per Mole. Solver for Moles Molar Mass and Grams This program solves for moles molar mass or grams when given the other two values. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. In grams per mole the characteristic molar mass of an element is equal to its atomic mass. The molecular weight of the compound determines the mass of 1 mole of the specific substance and the number of grams per mole of the compound.
 The Mole And Molar Masses Chemistry Activities From wisc.pb.unizin.org
The Mole And Molar Masses Chemistry Activities From wisc.pb.unizin.org
Calculate the molarity of each of the following solutionsa. 1221 mg KI in 1076 mL of solution. Reasonable that this number of atoms would equal about 2 mol. Figure 64 is a chart for determining what conversion factor is needed and Figure 65 is a flow diagram for the steps needed to perform a conversion. All the other convers I saw sucked so I made my own. Avogadros number is the number of atoms per mole of lithium and can be used to calculate the number of moles from the number of atoms.
041 mol of LiNO3 in 626 L of solutionb.
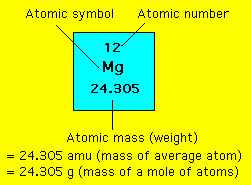
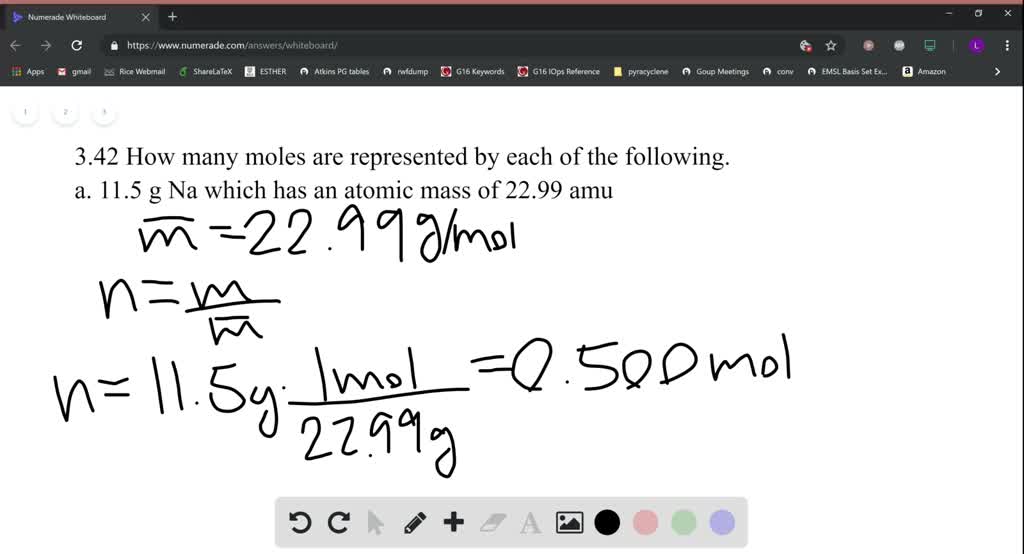
In other words the molar mass is the total mass in grams of all the atoms that make up one mole of a given molecule. In grams per mole the characteristic molar mass of an element is equal to its atomic mass. The Mole A mole abbreviated mol of a pure substance is a mass of the material in grams that is numerically equal to the molecular mass in atomic mass units amu. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. The chapter 3 science class 9 notes pdf explains the concept of substance which has mass and occupies space. Solver for Moles Molar Mass and Grams This program solves for moles molar mass or grams when given the other two values.
 Source: youtube.com
Source: youtube.com
The number 6022140761023 the Avogadro number was chosen so that the mass of one mole of a chemical compound in grams is numerically equal for most practical purposes to the average mass of one molecule of the compound in daltons. It describes 19th-century developments that led to the concept of the mole Topics include atomic weight molecular weight and molar mass. Solver for Moles Molar Mass and Grams This program solves for moles molar mass or grams when given the other two values. This module shows how the mole known as Avogadros number is key to calculating quantities of atoms and molecules. One mole of oxygen gas at STP is equal to____.

A hydrocarbon is composed of 8557 carbon and 1443 hydrogen and a. For example carbon has an atomic mass of exactly 120 atomic mass units – a mole of carbon is therefore 12 grams. Calculate the solubility in grams per literb. 022 x 10 23 molecules of oxygen b 6022 x 10 23 atoms of oxygen c 16 g of oxygen. A hydrocarbon is composed of 8557 carbon and 1443 hydrogen and a.
 Source: pediaa.com
Source: pediaa.com
They form a molecule per mole in an element. A hydrocarbon is composed of 8557 carbon and 1443 hydrogen and a. For example one atom of carbon has a mass of 12011 amu one mole of carbon has a mass of 12011 grams. G Mole is the amount of a substance containing elementary particles like atoms molecules or ions in 12 g of carbon-12. The atomic mass of potassium is 3910 amu and the atomic mass of bromine is 7990 amu.
 Source: slideplayer.com
Source: slideplayer.com
Atom has a mass of 55847 amu and 55847 g of iron contains 6022 137 1023 atoms of iron. A mole of any material will contain Avogadros number of molecules. The molecular weight is the number of grams per mole for the substance and gives the conversion factor for moles to grams for that particular substance. Ibuprofen a common pain remedy has an empirical formula of C_7H_9O and a molar mass of approximately 218078 grams per mole. A Molecules b Atoms c Ions d All of the above View Answer.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
The answer is 247. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. Conversions like this are possible for any substance as long as the proper atomic mass formula mass or molar mass is known or can be determined and expressed in grams per mole. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry. G Mole is the amount of a substance containing elementary particles like atoms molecules or ions in 12 g of carbon-12.
 Source: youtube.com
Source: youtube.com
It describes 19th-century developments that led to the concept of the mole Topics include atomic weight molecular weight and molar mass. Ibuprofen a common pain remedy has an empirical formula of C_7H_9O and a molar mass of approximately 218078 grams per mole. Figure 64 is a chart for determining what conversion factor is needed and Figure 65 is a flow diagram for the steps needed to perform a conversion. A joules per mole of nuclei b joules per nucleus c MeV per nucleus. The number 6022140761023 the Avogadro number was chosen so that the mass of one mole of a chemical compound in grams is numerically equal for most practical purposes to the average mass of one molecule of the compound in daltons.
 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
D 32 g of oxygen. Mole Convertor This program converts moles to grams grams to moles moles to atoms and atoms to moles. Determine the binding energy in joules per nuclide using the mass-energy equivalence equation. Per the amu definition a single 12 C atom weighs 12 amu its atomic mass is 12 amu. A Molecules b Atoms c Ions d All of the above View Answer.

This module shows how the mole known as Avogadros number is key to calculating quantities of atoms and molecules. Because the definitions of both the mole and the atomic mass unit are based on the same reference substance 12 C the molar mass of any substance is numerically equivalent to its atomic or formula weight in amu. 729 g C2H6O in 235 L of solutionc. Solver for Moles Molar Mass and Grams This program solves for moles molar mass or grams when given the other two values. Solution The mass defect for a latex_24textHelatex nucleus is 00305 amu as shown previously.
 Source: cpanhd.sitehost.iu.edu
Source: cpanhd.sitehost.iu.edu
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. 729 g C2H6O in 235 L of solutionc. It describes 19th-century developments that led to the concept of the mole Topics include atomic weight molecular weight and molar mass. The unit of molar volume is litre. Because the definitions of both the mole and the atomic mass unit are based on the same reference substance 12 C the molar mass of any substance is numerically equivalent to its atomic or formula weight in amu.

Sola d 1 mole of 0 2 gas at STP 6022 x 10 23 molecules of 0 2 Avogadro number 32 g of 0 2. Mole Convertor This program converts moles to grams grams to moles moles to atoms and atoms to moles. - 11900 amu The formula weight is the mass of one molecule or formula unit. The answer is 247. So one mole of water has a mass of 1802 grams 1 mol H2O x 1802 gmol 1802 g.
 Source: numerade.com
Source: numerade.com
For example one atom of carbon has a mass of 12011 amu one mole of carbon has a mass of 12011 grams. This can be calculated by adding the atomic mass of each atom. Per the amu definition a single 12 C atom weighs 12 amu its atomic mass is 12 amu. What is the molecular formula of ibuprofen. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
 Source: quora.com
Source: quora.com
Solution The mass defect for a latex_24textHelatex nucleus is 00305 amu as shown previously. Calculate the molarity of each of the following solutionsa. Here a molecular weight is the sum of the total mass in grams and atoms. The molecular weight of the compound determines the mass of 1 mole of the specific substance and the number of grams per mole of the compound. So one mole of water has a mass of 1802 grams 1 mol H2O x 1802 gmol 1802 g.

For example carbon has an atomic mass of exactly 120 atomic mass units – a mole of carbon is therefore 12 grams. Figure 64 is a chart for determining what conversion factor is needed and Figure 65 is a flow diagram for the steps needed to perform a conversion. What is the molecular weight of ibuprofen C13H18O2. Atom has a mass of 55847 amu and 55847 g of iron contains 6022 137 1023 atoms of iron. So one mole of water has a mass of 1802 grams 1 mol H2O x 1802 gmol 1802 g.
 Source: slideplayer.com
Source: slideplayer.com
How many grams Cm in 1 mol. What is the molecular weight of ibuprofen C13H18O2. What is grams per mole equal to. When we do stoichiometry we always want to speak about chemicals in terms of how many. This module shows how the mole known as Avogadros number is key to calculating quantities of atoms and molecules.

The atomic mass of potassium is 3910 amu and the atomic mass of bromine is 7990 amu. A joules per mole of nuclei b joules per nucleus c MeV per nucleus. 1 mol Li 6022 1023 atoms Li. 022 x 10 23 molecules of oxygen b 6022 x 10 23 atoms of oxygen c 16 g of oxygen. One mole of all gaseous substances at 273 K and 1 atm pressure occupies a volume equal to 224 litre or 22400 mL.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title does amu equal grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






