Your Converting molar mass to molality images are available. Converting molar mass to molality are a topic that is being searched for and liked by netizens now. You can Find and Download the Converting molar mass to molality files here. Get all free vectors.
If you’re searching for converting molar mass to molality images information linked to the converting molar mass to molality interest, you have visit the ideal blog. Our website frequently gives you suggestions for seeking the maximum quality video and image content, please kindly surf and locate more enlightening video articles and images that fit your interests.
Converting Molar Mass To Molality. Gmole Concentration of the substance. The number of grams of KClO3 will be 30637. Conversion from Molality to Molarity Problem. This aqueous solution has a density of 1101 gmL.
 Mole Conversions Made Easy How To Convert Between Grams And Moles Youtube Teaching Chemistry Mole Conversion Molar Mass From pinterest.com
Mole Conversions Made Easy How To Convert Between Grams And Moles Youtube Teaching Chemistry Mole Conversion Molar Mass From pinterest.com
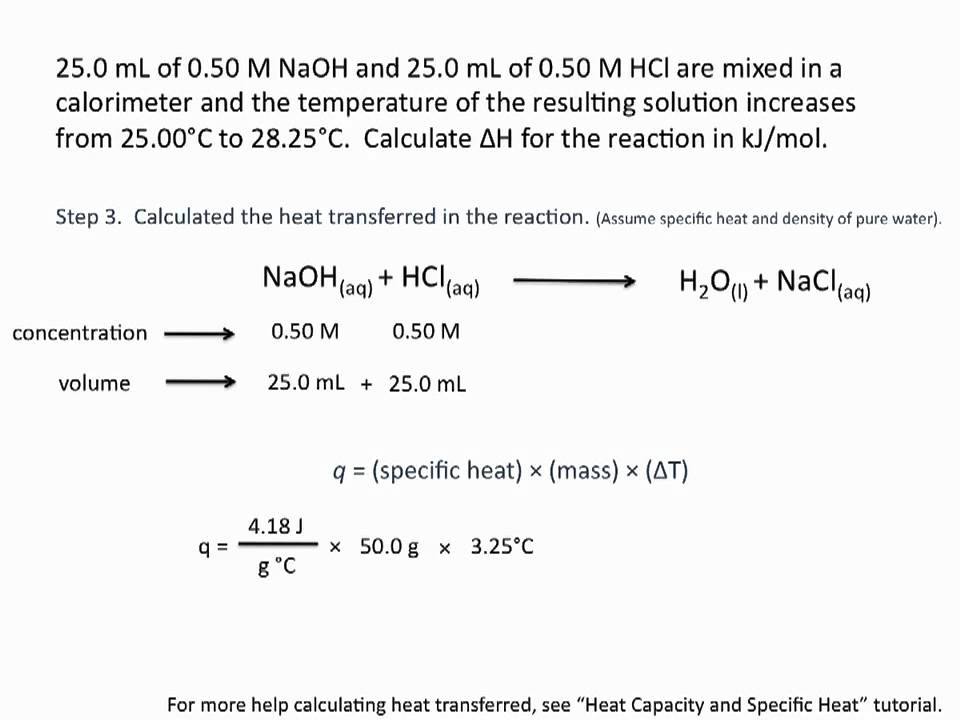
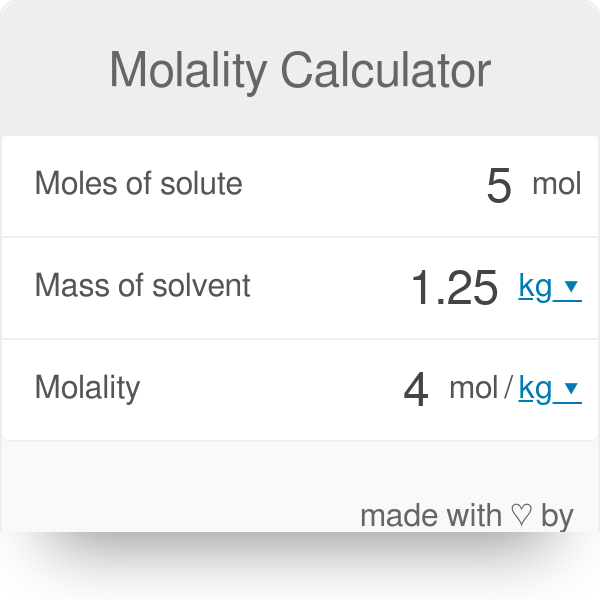
45 Convert molmL to molar - Conversion of Measurement Units. 257 Your goal here is to figure out the number of grams of solute present for every 100 g of the solution ie. Calculate the grams of the solvent. These concentration metrics are all related by relatively simple formulas Figure 1. Any input will be greatly appreciated. Molality moles solutekg solvent Ms mass of solvent in kg m mass of solute MM molar mass molality mMMMs MM mMsmolality.
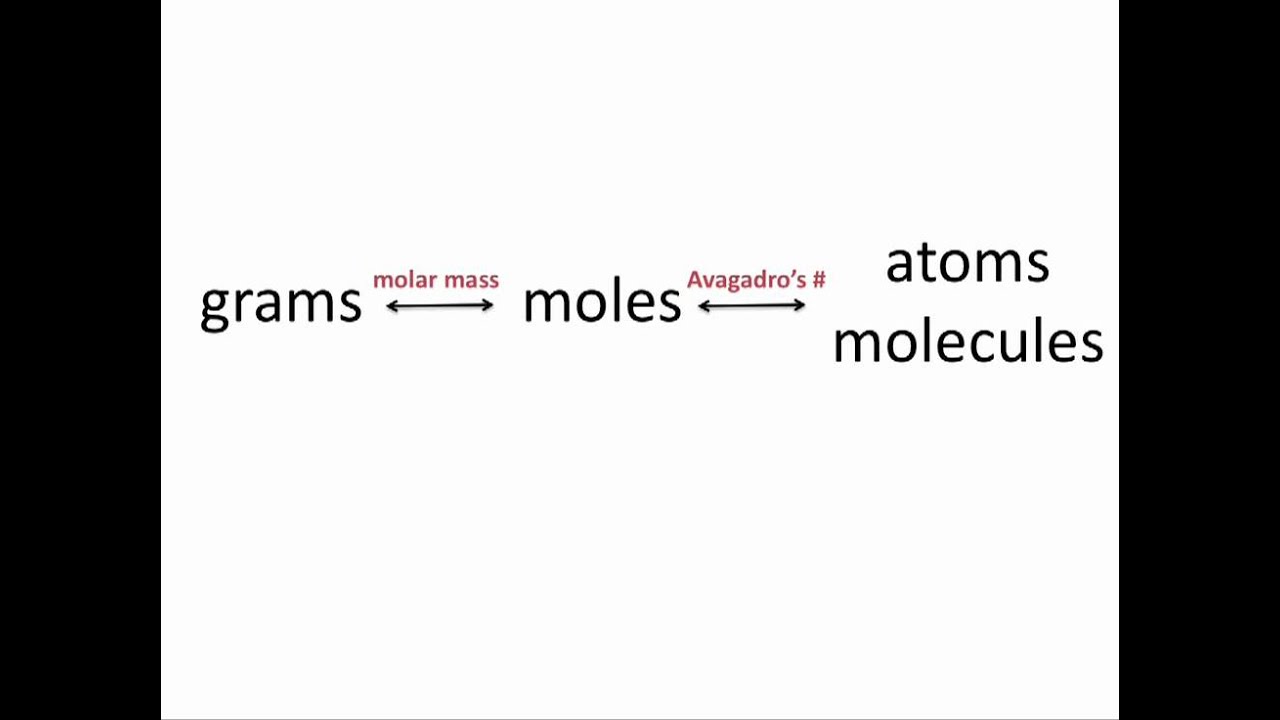
Use the molecular formula to find the molar mass.
Given these numbers how would I find the molarity. How to dissolve steel without affecting aluminium alloy. Given these numbers how would I find the molarity. Convert 18 moles to grams. This tells you that this solution contains 235 moles of rubidium nitrate the solute for every 1 kg of water the solvent. This is a very important step and the amount of solution is not given but you need to have a specific quantity to do the.
 Source: pinterest.com
Source: pinterest.com
The problem involves an aqueous solution of ammonium sulfate NH42SO4 with a molality of 311. Now we will calculate moles in solute from the molar mass. Assume you have 1 kg of solvent water. How do I calculate moles. Spanish French German Russian Italian Portuguese Polish Dutch.
 Source: pinterest.com
Source: pinterest.com
Let the volume of the solution be V. Calculate the grams of the solvent. Molar mass of KMnO 4 391 g 549 g 160 g x 4 Molar mass of KMnO 4 1580 g. To use this online calculator for Mole Fraction using Molality enter Molality m Molar Mass Of The Solvent M2 and hit the calculate button. Now you know that the solution has a molality equal to 235 mol kg-1.
 Source: pinterest.com
Source: pinterest.com
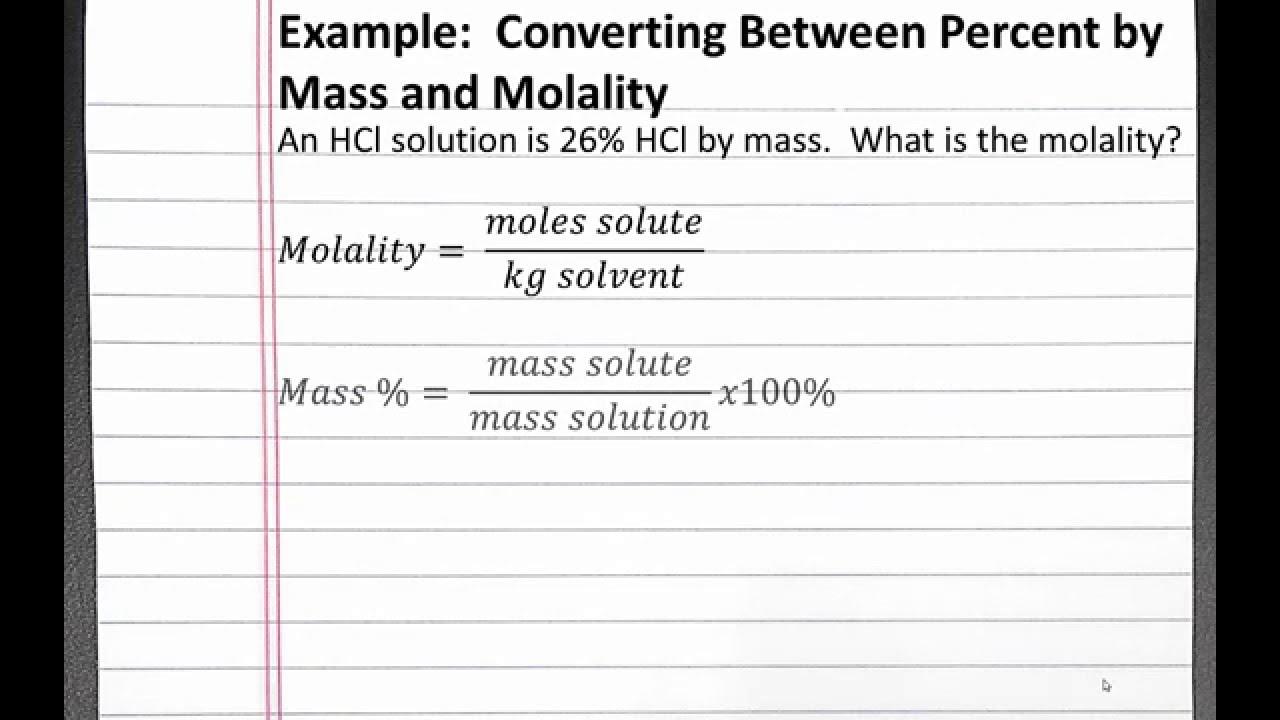
Reaction rate unit conversion. You can use Molar mass of the substance alone to calculate molar mass. Of course you can usually simplify this further by noting that DmV so molality is equal to the molarity divided by the solutions density. The solutions percent concentration by mass mm. Converting Percentage by Mass to Molarity.
 Source: omnicalculator.com
Source: omnicalculator.com
Molar volume allows conversions to be made between moles and volume of gases at STP. This aqueous solution has a density of 1101 gmL. Converting Percentage by Mass to Molarity. Find Molarity to molality converter at CalcTown. Molality moles solutekg solvent Ms mass of solvent in kg m mass of solute MM molar mass molality mMMMs MM mMsmolality.
 Source: youtube.com
Source: youtube.com
Gas density can be calculated from the molar mass and molar volume. Gas density can be calculated from the molar mass and molar volume. Let the molar mass of solute be M. Let the mass of given solute be W. Given these numbers how would I find the molarity.
 Source: pinterest.com
Source: pinterest.com
The solute is ammonia which is ceNH3 with a molar mass MM of pu17 g mol-1 whereas the solvent is water or ceH2O which has a molar mass of pu18 g mol-1. The pu200 m from the problem signifies molality because of. Now you know that the solution has a molality equal to 235 mol kg-1. These concentration metrics are all related by relatively simple formulas Figure 1. The solute is ammonia which is ceNH3 with a molar mass MM of pu17 g mol-1 whereas the solvent is water or ceH2O which has a molar mass of pu18 g mol-1.
 Source: pinterest.com
Source: pinterest.com
Molarity using Molality calculator uses molarity Density 1 Molality Molecular Mass Solute 1000 to calculate the Molarity The Molarity using Molality formula is defined as the number of moles of the solute dissolved in 1 liter of solution. The problem involves an aqueous solution of ammonium sulfate NH42SO4 with a molality of 311. Relation Between Molarity And Molality. To convert mgmL to molarity requires converting milligrams to grams and then using the molar mass of the solute to find the total number of moles. The solutions percent concentration by mass mm.
 Source: pinterest.com
Source: pinterest.com
Of course you can usually simplify this further by noting that DmV so molality is equal to the molarity divided by the solutions density. The density of the solution is 123 gmL. This aqueous solution has a density of 1101 gmL. I am having difficulty converting molality to molarity. Spanish French German Russian Italian Portuguese Polish Dutch.
 Source: pinterest.com
Source: pinterest.com
Molarity using Molality calculator uses molarity Density 1 Molality Molecular Mass Solute 1000 to calculate the Molarity The Molarity using Molality formula is defined as the number of moles of the solute dissolved in 1 liter of solution. Molarity is dependent on temperature. Substance molecular weight molar mass. 1840 grams of solution - 176562 grams of solute 7438 grams solvent. Let the molar mass of solute be M.
 Source: youtube.com
Source: youtube.com
The molar mass of sulfuric acid is 9809 gmol. Use our free online app Molarity to molality converter to determine all important calculations with parameters and constants. Gmole Concentration of the substance. There are various units which help us define Molality and. The volume of 1 mole of any gas is called its molar volume and is equal to 224 L at standard temperature and pressure.
 Source: pinterest.com
Source: pinterest.com
Determine the moles of unknown the solute from the molality of the solution and the mass of solvent in kilograms used to make the solution. How can I calculate molality from molarity. Molality moles solutekg solvent Ms mass of solvent in kg m mass of solute MM molar mass molality mMMMs MM mMsmolality. How to convert from molarity to ppm. I am having difficulty converting molality to molarity.
 Source: studylib.net
Source: studylib.net
Multiply the given number of moles 250 mol by the molar mass 122548 gmol to get the grams. The Molar mass of ammonium sulfate is 132gmol. Any input will be greatly appreciated. How to dissolve steel without affecting aluminium alloy. The number of grams of KClO3 will be 30637.
 Source: mathscinotes.com
Source: mathscinotes.com
Use our free online app Molarity to molality converter to determine all important calculations with parameters and constants. In this post I will review the formulas used to convert between the different electrolyte metrics. The problem involves an aqueous solution of ammonium sulfate NH42SO4 with a molality of 311. To use this online calculator for Mole Fraction using Molality enter Molality m Molar Mass Of The Solvent M2 and hit the calculate button. Molar mass C 12 H 22 O 11 12Atomic mass of C 22Atomic mass of H 11Atomic mass of O 121201 221008 111600 342296 gmol.
 Source: pinterest.com
Source: pinterest.com
The solute is ammonia which is ceNH3 with a molar mass MM of pu17 g mol-1 whereas the solvent is water or ceH2O which has a molar mass of pu18 g mol-1. Let the weight of the solvent be W. Molar volume allows conversions to be made between moles and volume of gases at STP. The pu200 m from the problem signifies molality because of. Use our free online app Molarity to molality converter to determine all important calculations with parameters and constants.
 Source: youtube.com
Source: youtube.com
Determine the moles of unknown the solute from the molality of the solution and the mass of solvent in kilograms used to make the solution. Of course you can usually simplify this further by noting that DmV so molality is equal to the molarity divided by the solutions density. Let the mass of given solute be W. 18M means 18 moles of sulfuric acid per one liter of solution. These concentration metrics are all related by relatively simple formulas Figure 1.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title converting molar mass to molality by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.