Your Convert molecular weight to molar mass images are available. Convert molecular weight to molar mass are a topic that is being searched for and liked by netizens now. You can Get the Convert molecular weight to molar mass files here. Get all royalty-free vectors.
If you’re searching for convert molecular weight to molar mass pictures information linked to the convert molecular weight to molar mass keyword, you have pay a visit to the ideal site. Our site always gives you suggestions for seeing the highest quality video and image content, please kindly surf and locate more informative video articles and graphics that fit your interests.
Convert Molecular Weight To Molar Mass. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. MGE Innovation Center 505 South Rosa Road Suite 238 Madison WI 53719. Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. Molar Mass conversion helps in converting different units of Molar Mass.
 Numeracy Maths And Statistics Academic Skills Kit From ncl.ac.uk
Numeracy Maths And Statistics Academic Skills Kit From ncl.ac.uk
The chemical formula should be entered using standard format. For example calcium carbonate would be entered as CaCO3 not caco3. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. Definitions of molecular mass molecular weight molar mass and molar weight. This feature simplifies many stoichiometric calculations. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100.
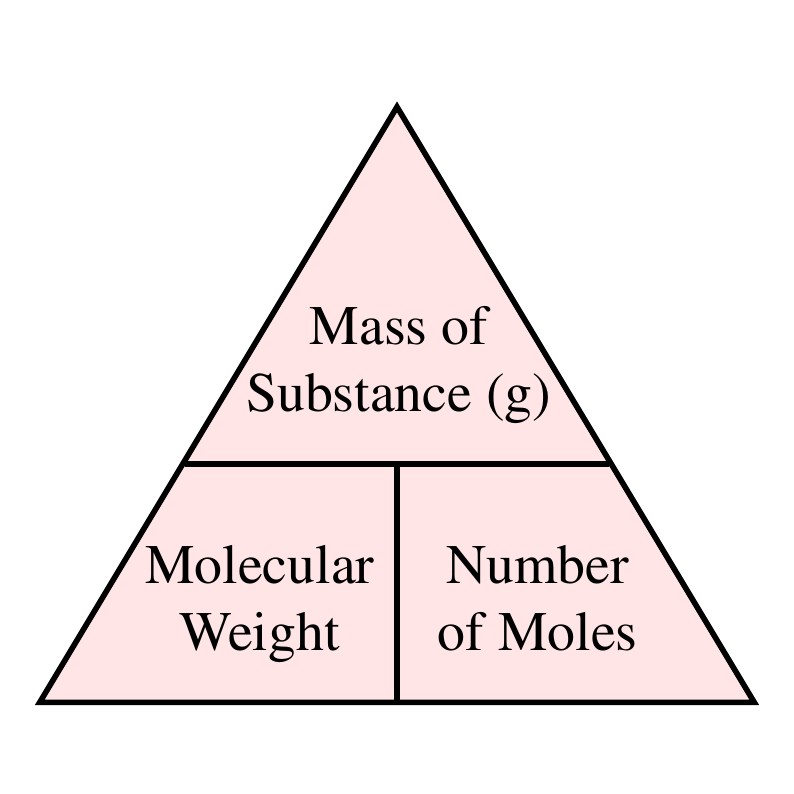
If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight.
For example the molecular formula of water is H 2 O. For example the water molecular weight is 18015 atomic mass units AMU so a mole of water weighs 18015 grams. If you have 125 grams of molecules with a molecular weight of 1341 gmol how many moles do you have. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. Nucleic Acid Molecular Weight Conversions Exact MW. Molar mass is the mass of one mole of a substance.
 Source: slideplayer.com
Source: slideplayer.com
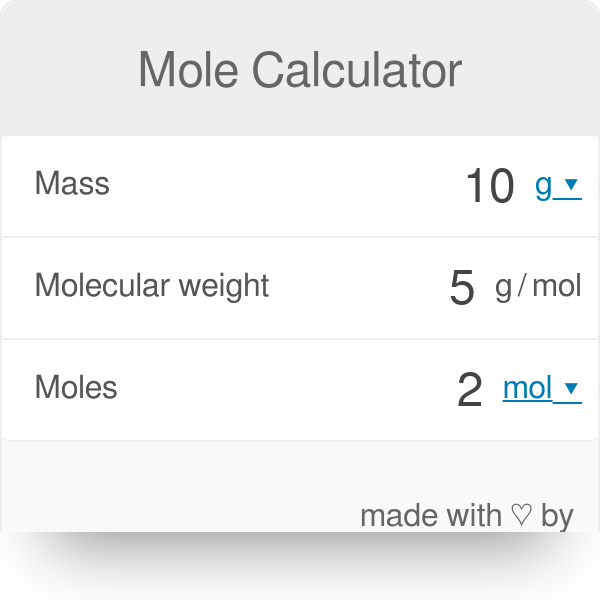
Mass g Concentration molL x Volume L x Molecular Weight gmol An example of a molarity calculation using the Tocris molarity calculator. How do you find molecular formula. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight. Molecular mass or molar mass are used in stoichiometry calculations in chemistry. Molecular mass calculator uses a molecular weight formula for finding molar mass.
 Source: omnicalculator.com
Source: omnicalculator.com
1 u is equal to 112 the mass of one atom of carbon-12. Of ssRNA eg RNA Transcript. You calculate molecular weight by taking the atomic weight and multiplying it by how many of that element you have in your molecule and then to add up each. Pricing Info Sample Submission Guidelines Submit Your Samples. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100.
 Source: youtube.com
Source: youtube.com
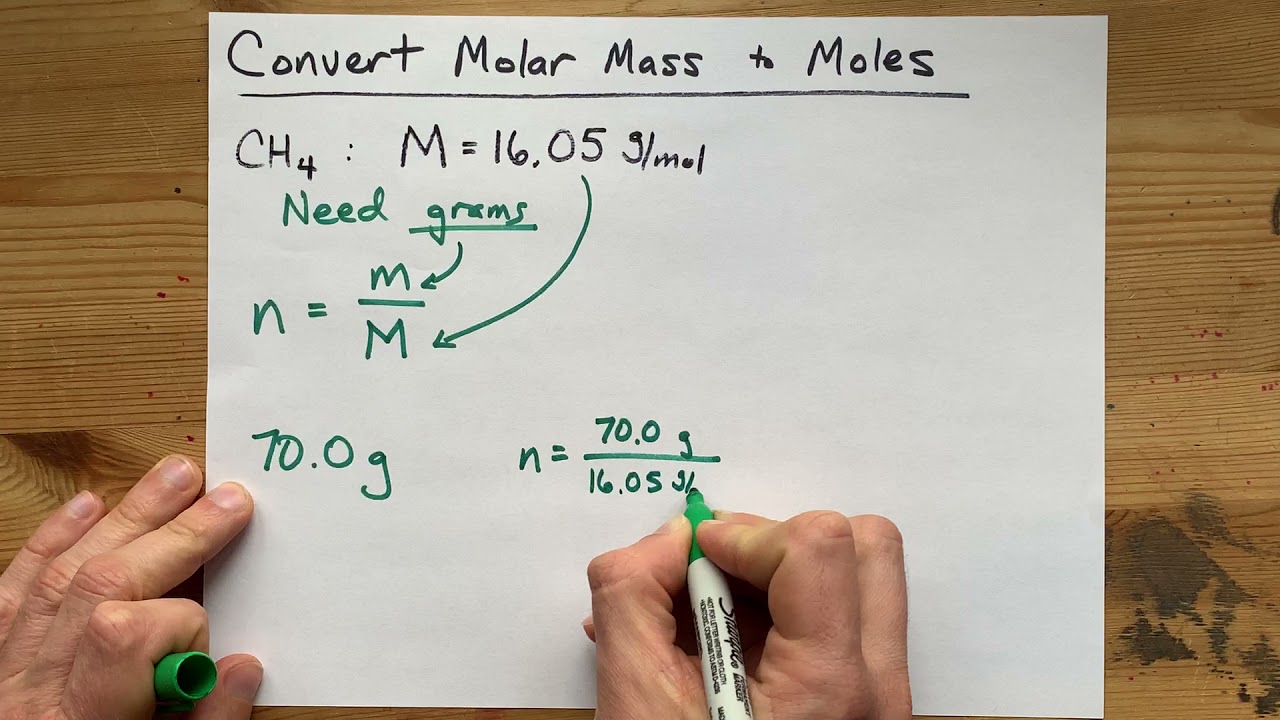
Therefore 0250 g 18017 amu 000139 moles of aspirin. If you have 125 grams of molecules with a molecular weight of 1341 gmol how many moles do you have. Nucleic Acid Molecular Weight Conversions Exact MW. The chemical formula should be entered using standard format. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100.
 Source: pinterest.com
Source: pinterest.com
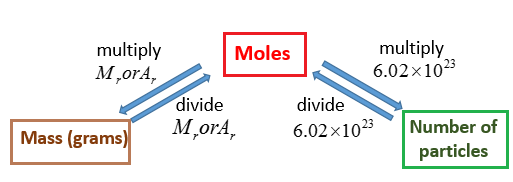
Molecular Weight number of hydrogen atomsH atomic weight number of oxygen atomsO atomic weight. The molecular weight formula is. Measurement units Molar Mass Conversion Molar Mass Converter Chlorine Cl - standard atomic weight Chlorine molecules Cl₂ - molecular mass Grams per molegmol Hydrogens H - standard atomic weight Hydrogen molecules H₂ - molecular mass Iron Fe - standard atomic weight Kilograms per molekgmol Oxygen O - standard atomic weight Sulfur S - standard. MGE Innovation Center 505 South Rosa Road Suite 238 Madison WI 53719. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
The molecular weight formula is. The molecular weight formula is. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight. Pricing Info Sample Submission Guidelines Submit Your Samples. Molecular mass calculator uses a molecular weight formula for finding molar mass.
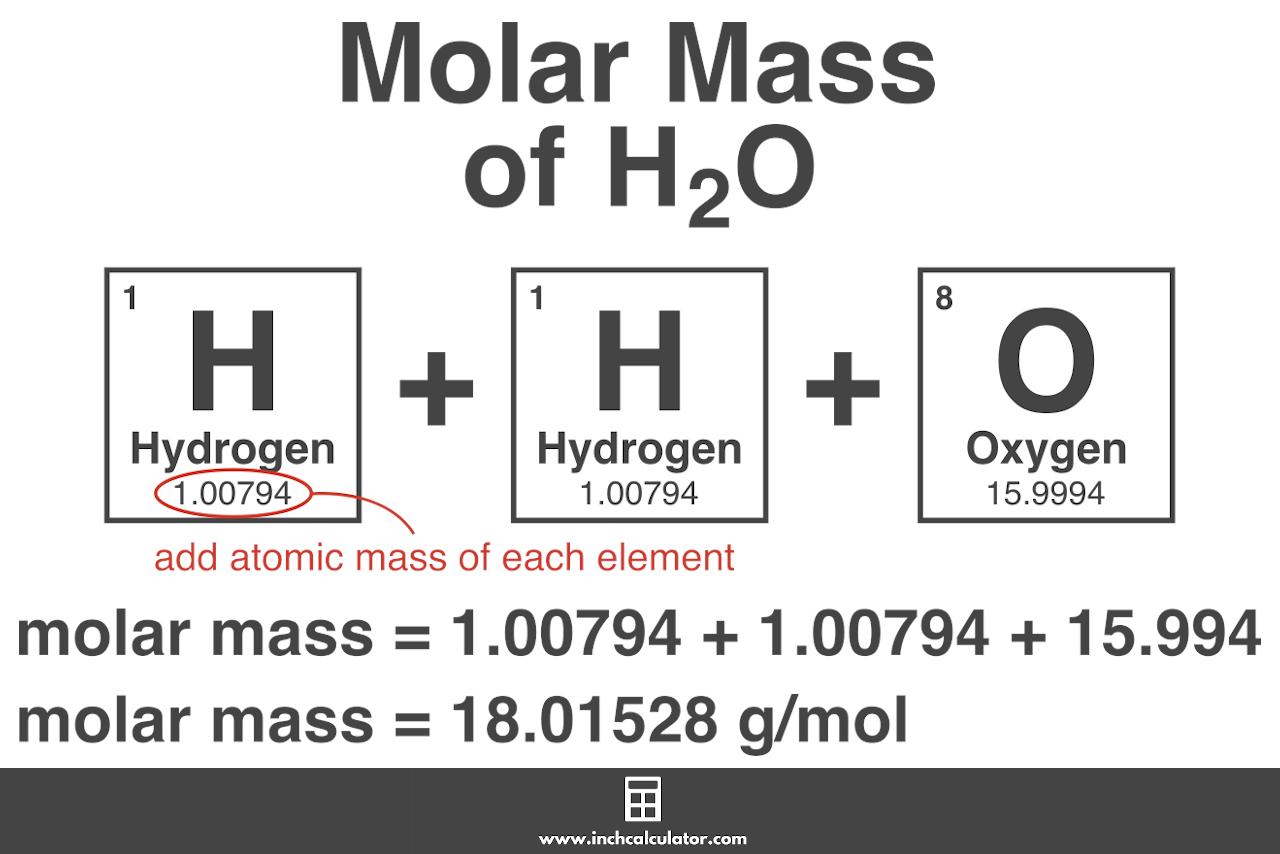 Source: inchcalculator.com
Source: inchcalculator.com
MGE Innovation Center 505 South Rosa Road Suite 238 Madison WI 53719. If you have 125 grams of molecules with a molecular weight of 1341 gmol how many moles do you have. 1 u is equal to 112 the mass of one atom of carbon-12. This feature simplifies many stoichiometric calculations. Weight to Molar Quantity for proteins This is used to convert the weight weight concentration into the molar quantity molar concentration for proteins and vice versa.
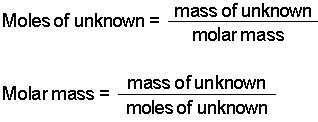 Source: chem.purdue.edu
Source: chem.purdue.edu
Therefore 0250 g 18017 amu 000139 moles of aspirin. How Molar Mass Calculator Works. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. Molecular mass calculator uses a molecular weight formula for finding molar mass. Molecular Weight number of hydrogen atomsH atomic weight number of oxygen atomsO atomic weight.
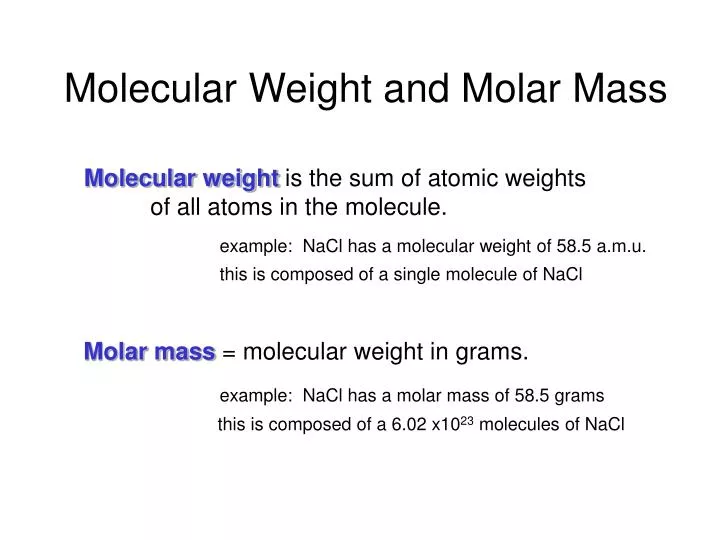 Source: slideserve.com
Source: slideserve.com
It is defined to be 112 of the mass of one atom of carbon-12 and in older works is also abbreviated as amu. What is the mass of compound required to make a 10 mM stock solution in 10 ml of water given that the molecular weight of the compound is 19713 gmol. Therefore 0250 g 18017 amu 000139 moles of aspirin. Molecular Weight To Moles - 9 images - molar mass molecular weight of n2o youtube how to convert grams to moles video lesson transcript. 1 u is equal to 112 the mass of one atom of carbon-12.
 Source: chemistrygod.com
Source: chemistrygod.com
The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. Calculate the moles of substance by dividing the mass of substance in grams by the molecular weight in amu. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight. MGE Innovation Center 505 South Rosa Road Suite 238 Madison WI 53719.
 Source: slideplayer.com
Source: slideplayer.com
Molecular mass calculator uses a molecular weight formula for finding molar mass. Enter 19713 into the Molecular Weight MW box. How do you find molecular formula. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight.
 Source: ncl.ac.uk
Source: ncl.ac.uk
Molecular mass or molar mass are used in stoichiometry calculations in chemistry. Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. For example the water molecular weight is 18015 atomic mass units AMU so a mole of water weighs 18015 grams. Protein molecular weight kDa. You calculate molecular weight by taking the atomic weight and multiplying it by how many of that element you have in your molecule and then to add up each.
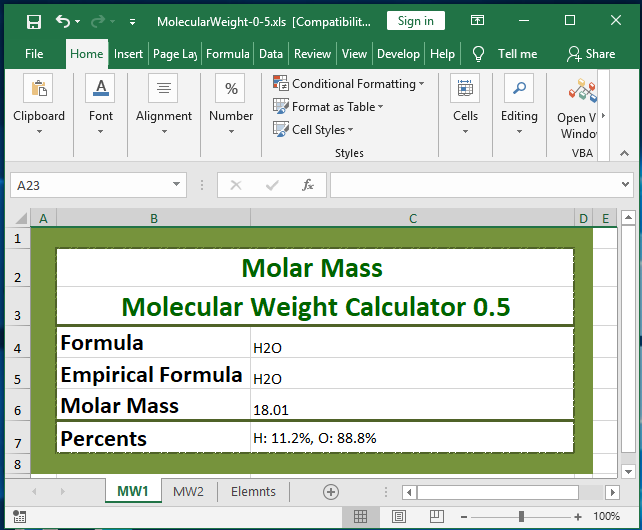 Source: miniindustry.com
Source: miniindustry.com
Weight to Molar Quantity for proteins This is used to convert the weight weight concentration into the molar quantity molar concentration for proteins and vice versa. Molecular Weight To Moles - 9 images - molar mass of a gas at stp equations formulas molar mass molecular weight of n2o youtube. What is the mass of compound required to make a 10 mM stock solution in 10 ml of water given that the molecular weight of the compound is 19713 gmol. Molecular mass calculator uses a molecular weight formula for finding molar mass. For example calcium carbonate would be entered as CaCO3 not caco3.
 Source: youtube.com
Source: youtube.com
Conversion of these quantities is equally important as measuring them. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight. Mass g Concentration molL x Volume L x Molecular Weight gmol An example of a molarity calculation using the Tocris molarity calculator. In this case the aspirin tablet contains 250 mg or 0250 g.
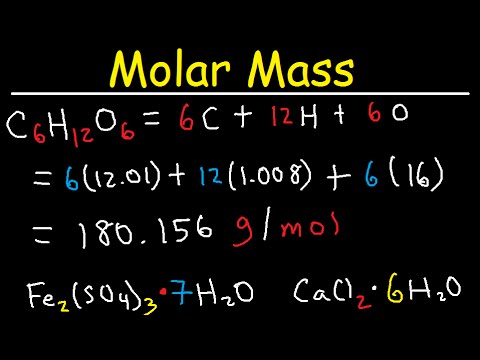 Source: youtube.com
Source: youtube.com
The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. Finding molar mass also called molecular weight molecular mass and gram formula mass is an essential skill in chemistry especially for mole to gram conv. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. Definitions of molecular mass molecular weight molar mass and molar weight. 1 u is equal to 112 the mass of one atom of carbon-12.
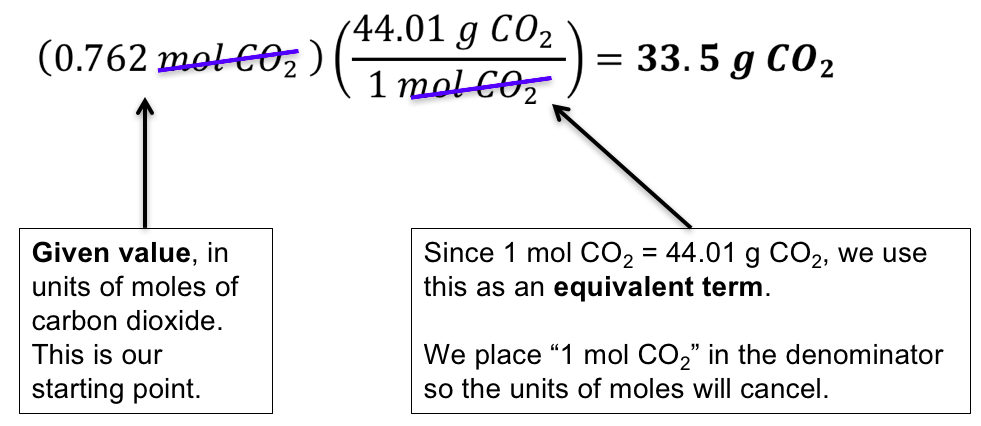 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. What is the mass of compound required to make a 10 mM stock solution in 10 ml of water given that the molecular weight of the compound is 19713 gmol. Protein molecular weight kDa. Examples of molecular weight computations. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title convert molecular weight to molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






