Your Conversion molarity to g l images are available in this site. Conversion molarity to g l are a topic that is being searched for and liked by netizens now. You can Get the Conversion molarity to g l files here. Get all royalty-free images.
If you’re looking for conversion molarity to g l images information connected with to the conversion molarity to g l interest, you have visit the right site. Our website frequently provides you with hints for downloading the highest quality video and picture content, please kindly surf and locate more informative video content and images that fit your interests.
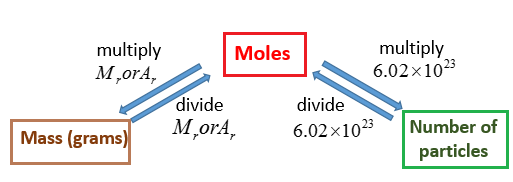
Conversion Molarity To G L. Convert gL to mgL. 0118 mol L-1 x 559 g mol-1 660 g L-1 3 sf How many grams are there in 1 cubic meter. You can view more details on each measurement unit. Its units are molL moldm 3 or molm 3.
 Mole Fraction Easy Science Mole Fraction Easy Science Fractions From pinterest.com
Mole Fraction Easy Science Mole Fraction Easy Science Fractions From pinterest.com
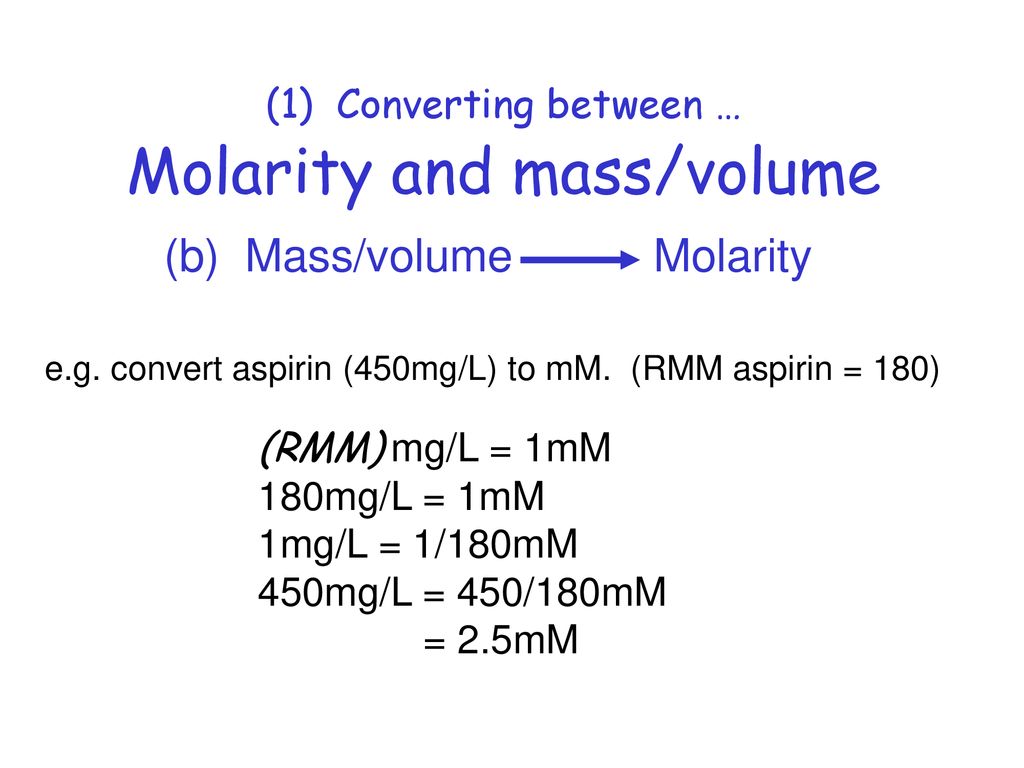
What is the equation to find grams per liter. The molar mass of a compound is the conversion factor between solubility and molar solubility. Molarity is MolL which when multiplied with the Molecular Mass sometimes also called Molecular Weight becomes gramsliter. The molar mass of an atom is its average atomic mass expressed in grams. To convert to grams you need to know what the substance is and its molar mass which is numerically equal to the molecular weight. Molar concentration is the amount of a solute present in one unit of a solution.
We assume you are converting between gramlitre and grammillilitre.
The density of the solution is 119 gmL. 0118 mol L -1 x 559 g mol-1 660 g L -1 3 sf Note. Multiply your grams per liter gL from the step above by the simple metric. What is the equation to find grams per liter. I think Im missing. To convert to g L-1 you multiply the concentration by the molar mass.
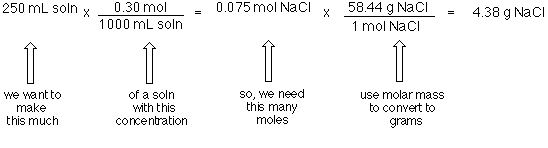 Source: westfield.ma.edu
Source: westfield.ma.edu
This solution has a density of 184 gmL. If you calculate this keeping all the figures in your calculator from step 4 you will get 658 g L -1 3 sf. X 850 gL. The molar mass of a compound is the conversion factor between solubility and molar solubility. Molarity is MolL which when multiplied with the Molecular Mass sometimes also called Molecular Weight becomes gramsliter.
 Source: youtube.com
Source: youtube.com
A gramme per litre or gram per liter gL or gl is a unit of measurement of mass concentration that shows how many grammes of a certain substance are present in one litre of a usually liquid or gaseous mixture. GL or gmL The SI derived unit for density is the kilogramcubic meter. Conversion from Molarity to Molality Problem. If I have 10 5 g L 1 C u X 2 solution do I have 157 10 7 m o l L 1. The molar mass of an atom is its average atomic mass expressed in grams.
 Source: slideplayer.com
Source: slideplayer.com
Parts per million concentrations are essentially mass ratios solute to solution x a million 106. Convert gL to mgL. Its units are molL moldm 3 or molm 3. This is generally true for freshwater and other dilute aqueous solutions. X 850 gL.
 Source: wikihow.com
Source: wikihow.com
Note that rounding errors may occur so always check the results. Once the number of grams of solute per liter is known the molarity can be calculated. This makes it a little easier because you dont need to convert between milligrams and grams or milliliters and liters. To prepare 1 L of 05 M sodium chloride solution then as per the formula use 2922 g of sodium chloride 05 molL 1L 5844 gmol. The unit of moles will cancel and you will be left with a measure of grams per liter gL.
 Source: pinterest.com
Source: pinterest.com
GL grams per liter mass of solute volume of solution F formality. If you calculate this keeping all the figures in your calculator from step 4 you will get 658 g L -1 3 sf. Parts per million concentrations are essentially mass ratios solute to solution x a million 106. GL grams per liter mass of solute volume of solution F formality. Calculate the mass of 1 L of solution.
 Source: pinterest.com
Source: pinterest.com
Since molarity is defined in terms of gL convert 085 g100 mL to gL. I think Im missing. Molar concentration also known as molarity and can be denoted by the unit M molar. Multiply your grams per liter gL from the step above by the simple metric. Assume you have 1 L of solution.
 Source: pinterest.com
Source: pinterest.com
X 850 gL. We use molar mass which is grams per mole. Its units are molL moldm 3 or molm 3. With the concentration in gL you can then convert grams to moles the molar mass. 0118 mol L-1 x 559 g mol-1 660 g L-1 3 sf What does G L measure.
 Source: omnicalculator.com
Source: omnicalculator.com
I know I should divide g L 1 by the molar mass of the substance but I dont seem to find the specific answer on Google. Its units are molL moldm 3 or molm 3. Multiply your grams per liter gL from the step above by the simple metric. Molar concentration is the amount of a solute present in one unit of a solution. The answer is 1000.
 Source: researchgate.net
Source: researchgate.net
Molarity is MolL which when multiplied with the Molecular Mass sometimes also called Molecular Weight becomes gramsliter. The molar mass of a compound is the conversion factor between solubility and molar solubility. Since 1 mg 10-3 g and 1 kg 103 g. We assume you are converting between gramlitre and grammillilitre. I know I should divide g L 1 by the molar mass of the substance but I dont seem to find the specific answer on Google.
 Source: pinterest.com
Source: pinterest.com
This solution has a density of 184 gmL. Multiply your grams per liter gL from the step above by the simple metric. A gramme per litre or gram per liter gL or gl is a unit of measurement of mass concentration that shows how many grammes of a certain substance are present in one litre of a usually liquid or gaseous mixture. GL or gmL The SI derived unit for density is the kilogramcubic meter. Note that rounding errors may occur so always check the results.
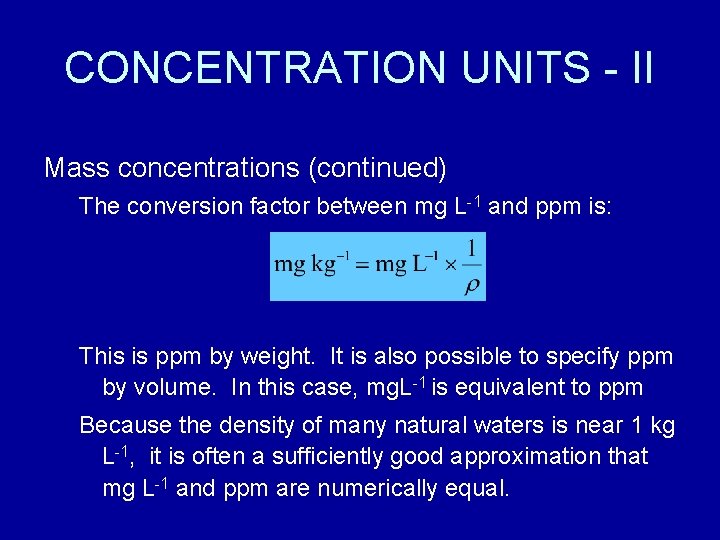 Source: slidetodoc.com
Source: slidetodoc.com
The number of gramsMol. The number of gramsMol. What is its molar concentration. Youll need to know the volume of water used. So just to be sure.
 Source: in.pinterest.com
Source: in.pinterest.com
What is the equation to find grams per liter. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide. The atomic mass of all atoms can be found in the periodic table. With the concentration in gL you can then convert grams to moles the molar mass. Its units are molL moldm 3 or molm 3.
 Source: wikihow.com
Source: wikihow.com
In this sense they are similar to wt which could be thought of as. Molarity refers to the molar concentration of a solution that is the number of moles of solute dissolved in 1 liter of solution as molL abbreviated as M. You can view more details on each measurement unit. To convert to grams you need to know what the substance is and its molar mass which is numerically equal to the molecular weight. Now we are already in litres so how to convert moles to grams.
 Source: tr.pinterest.com
Source: tr.pinterest.com
In other words the molar solubility of a given compound represents the highest molarity solution that is possible for that compound. To convert to grams you need to know what the substance is and its molar mass which is numerically equal to the molecular weight. In other words the molar solubility of a given compound represents the highest molarity solution that is possible for that compound. We use molar mass which is grams per mole. How do you convert mol L to G.
 Source: youtube.com
Source: youtube.com
0118 mol L-1 x 559 g mol-1 660 g L-1 3 sf What does G L measure. 1 kilogramcubic meter is equal to 1 gL or 0001 gmL. Given that the solubility of ZnOH 2 is 42 10 -4 gL the molar solubility can be calculated as shown below. How do you convert mol L to G. The answer is 1000.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title conversion molarity to g l by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.