Your Carbon grams to moles calculator images are available in this site. Carbon grams to moles calculator are a topic that is being searched for and liked by netizens today. You can Get the Carbon grams to moles calculator files here. Download all royalty-free images.
If you’re looking for carbon grams to moles calculator pictures information linked to the carbon grams to moles calculator interest, you have pay a visit to the ideal blog. Our site frequently gives you hints for refferencing the maximum quality video and image content, please kindly hunt and find more informative video articles and graphics that fit your interests.
Carbon Grams To Moles Calculator. One mole abbreviated mol is equal to the number of atoms in 12 grams of carbon-12. Do a quick conversion. What is cool about choosing the number we did for moles - all our 1 mole amounts match the molecular weight of the molecule in grams. Now it should be converted in terms of atoms.
 Moles From chemistry.wustl.edu
Moles From chemistry.wustl.edu
To calculate or find the grams to moles or moles to grams the molar mass of each element will be used to calculate. This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of carbon 120107 grams per mole of oxygen 159994 grams per mole and of chlorine 35453 grams per mole. Do a quick conversion. You can also verify the results by putting the values in free grams to molecules calculator. The molecular formula for Carbon Dioxide is CO2. The molecular mass of H 2 O is 18gmol.
1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023.
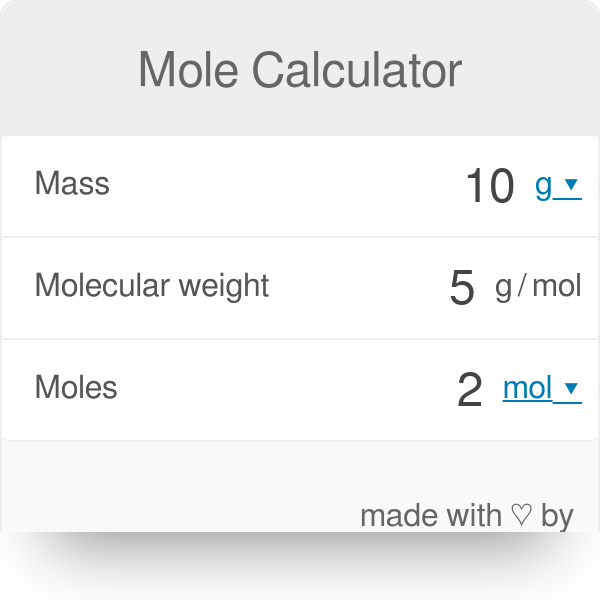
The mole calculator uses grams to moles formula to get actual results. 1 grams of carbon 0083259093974539 mole using the molecular weight calculator and the molar mass of C. What is cool about choosing the number we did for moles - all our 1 mole amounts match the molecular weight of the molecule in grams. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. 8 moles Carbon Tetraiodide to grams 415702864 grams. 1 grams Carbon Dioxide is equal to 0022722366761722 mole.
 Source: youtube.com
Source: youtube.com
For example imagine you have 2 g of water or H 2 O and you want to convert it to moles. 5 moles Carbon Tetraiodide to grams 25981429 grams. Now it should be converted in terms of atoms. Concentration Calculator makes it easy for you to determine the theoretical yield value of the chemical reaction in fraction of seconds with steps. Also the Avogadro constant is 602 x 10 23.
 Source: youtube.com
Source: youtube.com
Gap the mass of every component by the molar mass and increase the outcome by 100. The molecular mass of H 2 O is 18gmol. One mole abbreviated mol is equal to the number of atoms in 12 grams of carbon-12. Calculate the number of grams of Fe2O3 needed to react with 138 grams of carbon in the following reaction. The mole calculator uses grams to moles formula to get actual results.
 Source: pinterest.com
Source: pinterest.com
Burning one mole of octane 114 grams therefore would produce eight moles of CO2 with a weight of 352 grams 8 x 44. Using a calculator divide the number of grams by the molar mass. 5 Litres of Water 5000 ml of water. 8 moles Carbon Tetraiodide to grams 415702864 grams. N m M.
 Source: pinterest.com
Source: pinterest.com
What is cool about choosing the number we did for moles - all our 1 mole amounts match the molecular weight of the molecule in grams. 1 grams Carbon Disulfide 0013133580332201 mole using the molecular weight calculator and the molar mass of CS2. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. 4 moles Carbon Tetraiodide to grams 207851432 grams. You can also verify the results by putting the values in free grams to molecules calculator.
 Source: slideplayer.com
Source: slideplayer.com
2019-7-17 This is defined as 0001 kilogram per mole or 1 gram per mole. 2019-7-17 This is defined as 0001 kilogram per mole or 1 gram per mole. You can also verify the results by putting the values in free grams to molecules calculator. N m M. For calculations tap Molar Mass Calculator By using moles to grams formula.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
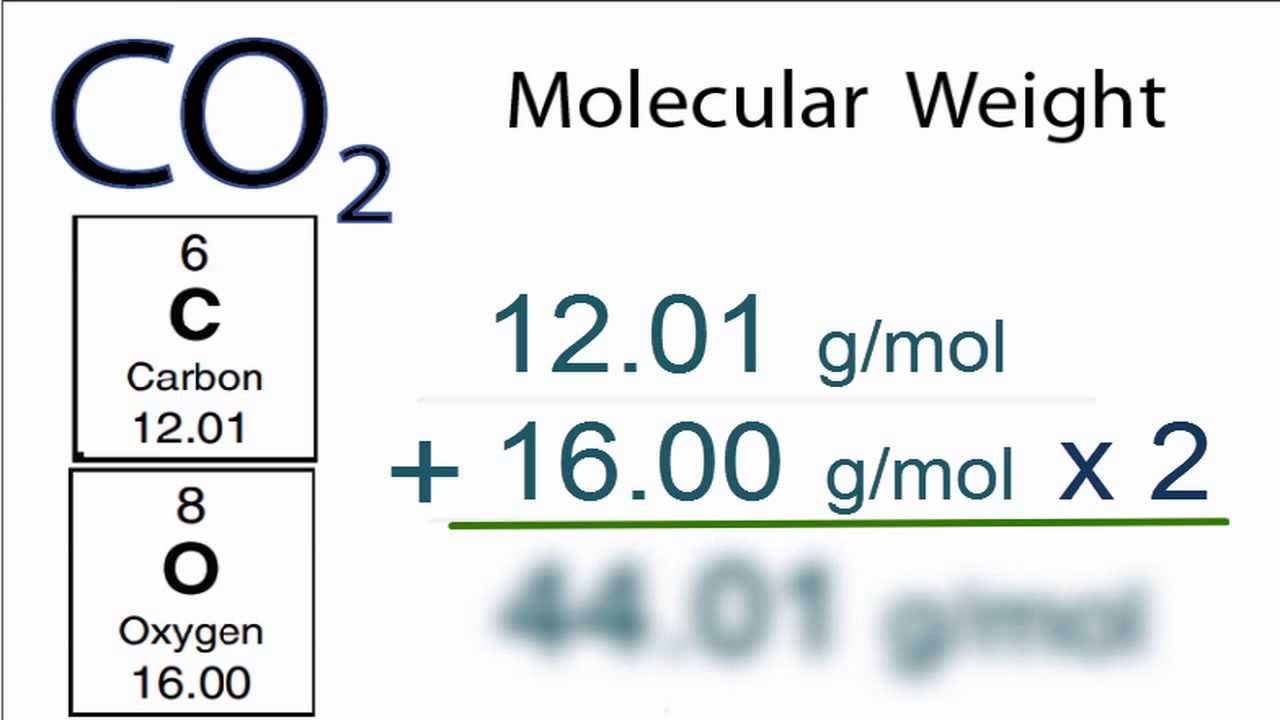
M 3899 107868. Using a calculator divide the number of grams by the molar mass. N m M. One mole abbreviated mol is equal to the number of atoms in 12 grams of carbon-12. The weight of CO2 is 44 grams per mole 1 x 12 gramsmole for the carbon and 2 x 16 gramsmole for the oxygen atoms.

It means that 12 grams per mole. So we chemists think in moles of substance. We know that the molar mass of 1 mole of carbon is equal to 12. Do a quick conversion. Grams to Moles Formula.
 Source: omnicalculator.com
Source: omnicalculator.com
3 moles Carbon Tetraiodide to grams 155888574 grams. 2019-7-17 This is defined as 0001 kilogram per mole or 1 gram per mole. They are only given to. Therefore 10 grams of carbon is equal to 502 x 10 23. Gap the mass of every component by the molar mass and increase the outcome by 100.
 Source: slideplayer.com
Source: slideplayer.com
The result is the number of moles in your element or compound. N 55503 m o l e s. The mole calculator uses grams to moles formula to get actual results. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of carbon 120107 grams per mole of oxygen 159994 grams per mole and of chlorine 35453 grams per mole.
 Source: pinterest.com
Source: pinterest.com
Calculate the number of grams of Fe2O3 needed to react with 138 grams of carbon in the following reaction. 1 grams Carbon 0083259093974539 mole using the molecular weight calculator and the molar mass of C. It means that 12 grams per mole. Therefore 10 grams of carbon is equal to 502 x 10 23. 5 Litres of Water 5000 ml of water.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
To calculate or find the grams to moles or moles to grams the molar mass of each element will be used to calculate. Total 121616 44 11 grams44 gramsmole025 moles of carbon The grams of water and combustion of 75 grams are totally irrelevant. Do a quick conversion. Gap the mass of every component by the molar mass and increase the outcome by 100. M 3899 107868.
 Source: slideplayer.com
Source: slideplayer.com
The answer is 440095. Also the Avogadro constant is 602 x 10 23. We know that the molar mass of 1 mole of carbon is equal to 12. This number is referred to as Avogadros number and has been measured as approximately 6022times 1023. You can view more details on each measurement unit.
 Source: pinterest.com
Source: pinterest.com
Carrying out grams to moles conversion. 12 g of CarbonC 1 Mole of Carbon C 602214076 1023 number of Atoms 635 g of Copper Cu 1 Mole of Copper Cu 602214076 1023 number of Atoms How Many Molecules in a Mole. The mole calculator uses grams to moles formula to get actual results. Do a quick conversion. What is cool about choosing the number we did for moles - all our 1 mole amounts match the molecular weight of the molecule in grams.

You can also verify the results by fetching the. The answer is 440095. Do a quick conversion. 1 grams of carbon 0083259093974539 mole using the molecular weight calculator and the molar mass of C. 12 g of CarbonC 1 Mole of Carbon C 602214076 1023 number of Atoms 635 g of Copper Cu 1 Mole of Copper Cu 602214076 1023 number of Atoms How Many Molecules in a Mole.
 Source: youtube.com
Source: youtube.com
We count in moles. One mole abbreviated mol is equal to the number of atoms in 12 grams of carbon-12. 1 grams Carbon 0083259093974539 mole using the molecular weight calculator and the molar mass of C. Some elements are only found in molecules of 2 atoms or more. What is cool about choosing the number we did for moles - all our 1 mole amounts match the molecular weight of the molecule in grams.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title carbon grams to moles calculator by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






