Your Calculate the hydrogen ion concentration in moles images are available in this site. Calculate the hydrogen ion concentration in moles are a topic that is being searched for and liked by netizens today. You can Find and Download the Calculate the hydrogen ion concentration in moles files here. Find and Download all royalty-free vectors.
If you’re searching for calculate the hydrogen ion concentration in moles pictures information connected with to the calculate the hydrogen ion concentration in moles topic, you have come to the right blog. Our website frequently gives you suggestions for viewing the maximum quality video and picture content, please kindly hunt and locate more enlightening video content and graphics that fit your interests.
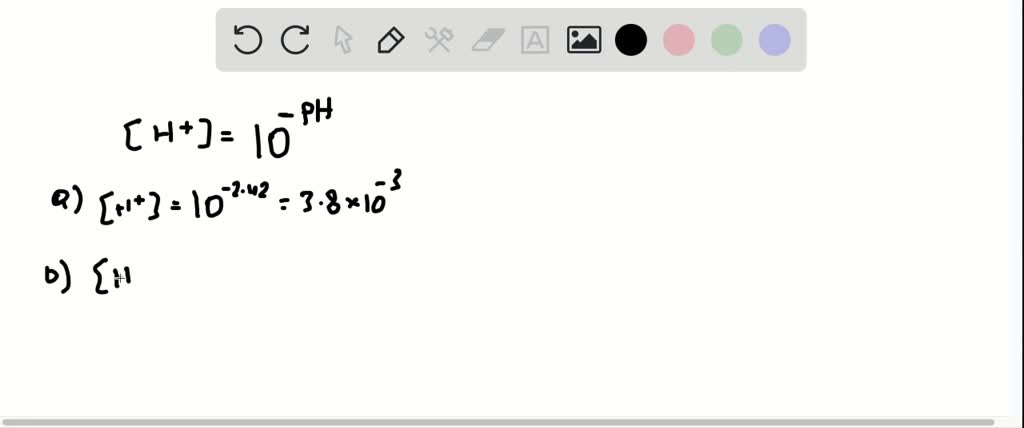
Calculate The Hydrogen Ion Concentration In Moles. A base is dissolved in water yields a solution with a hydroxide ion concentration of 005 mol litre 1. PH of a solution is calculated by logarithm with base 10 of the mol concentration of ion Hydrogen H of that solution. Calculate the hydrogen ion concentration in moles per liter for each of these solution. B Calculate the concentration of hydrogen ion in moles per litre of a solution whose pH is 54 The pH of the solution is 4.
 Basic Solution Easy Science Flashcards Solutions Easy Science From pinterest.com
Basic Solution Easy Science Flashcards Solutions Easy Science From pinterest.com
To use the 10 x button to calculate H you will need to. B X concentration loss by Mg2 ion. The pH is then calculated using the expression. A The pH of the solution is given as 520. What should be the change in the hydrogen ion concentration of the solution if its pH is to be increased to 50. Calculate the hydrogen ion concentration in moles per liter for a solution with a pOH of 429.
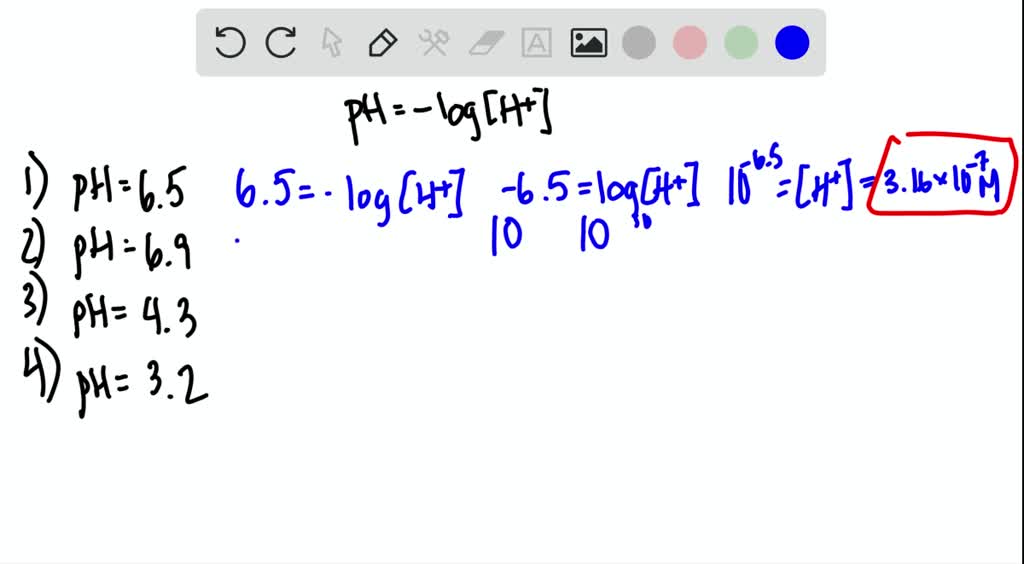
SOLVEDCalculate the hydrogen ion concentration in moles per liter for solutions with each of the following pH values.
In chemistry pH is a measure of the acidity or basicity of an aqueous solution. The hydrogen ion concentration of solution in mollitre is. Therefore the hydrogen ion concentration of a solution with pH 242 is. Round answer to one decimal place if necessary. Use your calculated value of number of OH - particles to find pOH and compare your value to that given in the question. B Calculate the concentration of hydrogen ion in moles per litre of a solution whose pH is 54 The pH of the solution is 4.
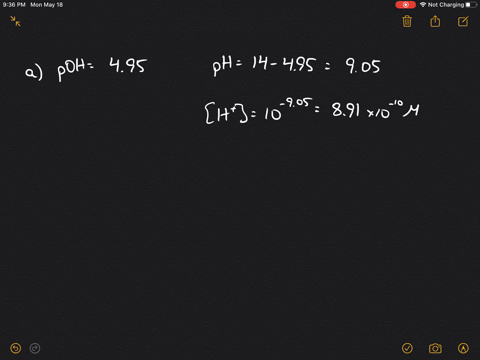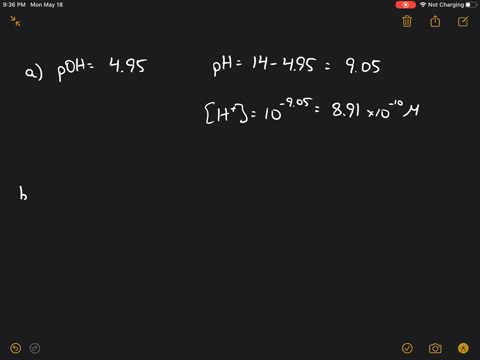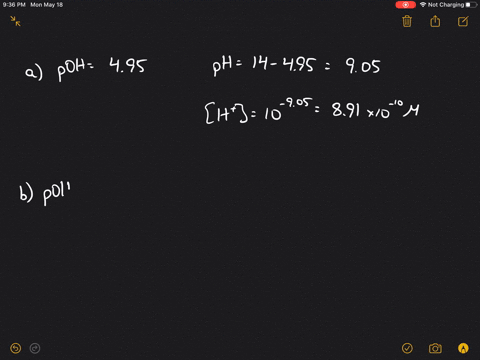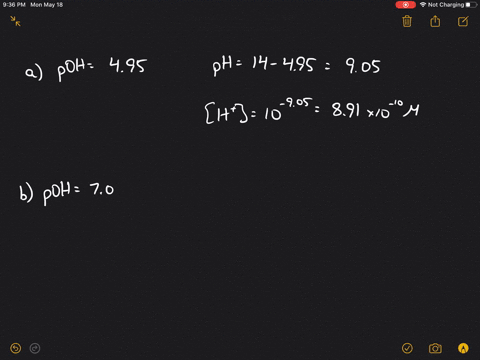 Source: numerade.com
Source: numerade.com
B Calculate the concentration of hydrogen ion in moles per litre of a solution whose pH is 54 The pH of the solution is 4. Ksp 709 10-9 121 10-3 - X01002. The hydrogen ion concentration is given by. Calculate the hydrogen ion concentration in moles per liter for a solution with a pOH of 429. What should be the change in the hydrogen ion concentration of the solution if its pH is to be increased to 50.
 Source: pinterest.com
Source: pinterest.com
So in this case tend to the negative 35 Will equal are proton concentration which will equal 0316 Mohler All right so for the 3rd 1 you know the the proton concentration will be content to negative 62 not legal. With our tool you need to enter the respective. So in this case tend to the negative 35 Will equal are proton concentration which will equal 0316 Mohler All right so for the 3rd 1 you know the the proton concentration will be content to negative 62 not legal. In chemistry pH is a measure of the acidity or basicity of an aqueous solution. B X concentration loss by Mg2 ion.
 Source: toppr.com
Source: toppr.com
Calculating H Focus From Ka Youtube Hydrogen Ion Focus From Ph Calculation Workthrough A2 Chemistry Youtube The Focus Of Hydrogen Ion In A Pattern Of Tender Drink Is. H concentration of acid is depended on its pKa for strong acid like HCl its pKa1 thus H concentration of 1 M HCl is also 1 M. X 12092914 10-3 Mg2 121 10-3. The hydrogen ion concentration is given by. To use the 10 x button to calculate H you will need to.
 Source: numerade.com
Source: numerade.com
PH of a solution is calculated by logarithm with base 10 of the mol concentration of ion Hydrogen H of that solution. Moles N N A 60 10 16 602 10 23 10 -7 mol. Using the equation pH log H we can solve for H as pH log H H 10 pH by exponentiating both sides with base 10 to undo the common logarithm. A The pH of the solution is given as 520. The hydrogen ion concentration is given by.

A pH 894 b pH 678 c pH 101 d pH 699. PH Hydrogen Ion Concentration Calculator. PH of a solution is calculated by logarithm with base 10 of the mol concentration of ion Hydrogen H of that solution. Multiply the pH by -1 to find -pH. Using the equation pH log H we can solve for H as pH log H H 10 pH by exponentiating both sides with base 10 to undo the common logarithm.
 Source: youtube.com
Source: youtube.com
A pH 894 b pH 678 c pH 101 d pH 699. A pH 894 b pH 678 c pH 101 d pH 699. A hydrogen ion concentration in a solution results from the addition of an acid. POH -log 10 OH - -log 10 10. In this problem we are given pH and asked to solve for the hydrogen ion concentration.
 Source: numerade.com
Source: numerade.com
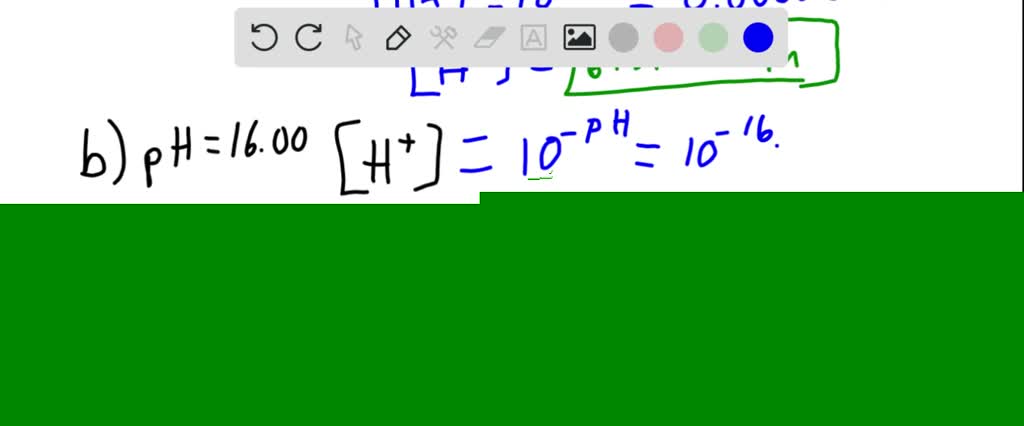
The pH of the solution is 4. The hydrogen ion concentration of an aqueous solution of a weak acid depends on the value of its acid dissociation constant and is always less than the concentration of the weak acid. Calculate the hydrogen ion concentration in moles per liter for solutions with each of the following pH values. A hydrogen ion concentration in a solution results from the addition of an acid. A a solution whose pH is 520 b a solition whose pH is 1600 c a soltuion whose hydroxide concentration is 37 X 10 M.
 Source: pinterest.com
Source: pinterest.com
Calculate the hydrogen ion concentration in moles per liter for each of these solution. Moles N N A 60 10 16 602 10 23 10 -7 mol. With our tool you need to enter the respective. The pH of the solution is 4. The pH of a solution is given by the formula pH -log H where H is the concentration of hydrogen ions in moles per liter in the solution.
 Source: numerade.com
Source: numerade.com
The hydrogen ion concentration of solution in mollitre is. What should be the change in the hydrogen ion concentration of the solution if its pH is to be increased to 50. The pH of a solution is 40. Calculate the hydrogen ion concentration in mole. Click the log button.

Using the equation pH log H we can solve for H as pH log H H 10 pH by exponentiating both sides with base 10 to undo the common logarithm. So in this case tend to the negative 35 Will equal are proton concentration which will equal 0316 Mohler All right so for the 3rd 1 you know the the proton concentration will be content to negative 62 not legal. The pH of a solution is given by the formula pH -log H where H is the concentration of hydrogen ions in moles per liter in the solution. Calculate the hydrogen ion concentration in mole. Ksp 709 10-9 121 10-3 - X01002.
 Source: bartleby.com
Source: bartleby.com
To use the 10 x button to calculate H you will need to. The pH of a solution is given by the formula pH -log H where H is the concentration of hydrogen ions in moles per liter in the solution. The hydrogen ion concentration of blood with pH 74 is. Click the INV button. Ksp 709 10-9 121 10-3 - X01002.
 Source: youtube.com
Source: youtube.com
The hydrogen ion concentration of solution in mollitre is. With our tool you need to enter the respective. A hydrogen ion concentration in a solution results from the addition of an acid. Click the INV button. In this problem we are given pH and asked to solve for the hydrogen ion concentration.
 Source: pinterest.com
Source: pinterest.com
The pH of a solution is given by the formula pH -log H where H is the concentration of hydrogen ions in moles per liter in the solution. The hydrogen ion concentration of blood with pH 74 is. With our tool you need to enter the respective. OH - moles volume in litres 10 -7 100mL1000mLL 10 -6 mol L -1. Multiply the pH by -1 to find -pH.
 Source: numerade.com
Source: numerade.com
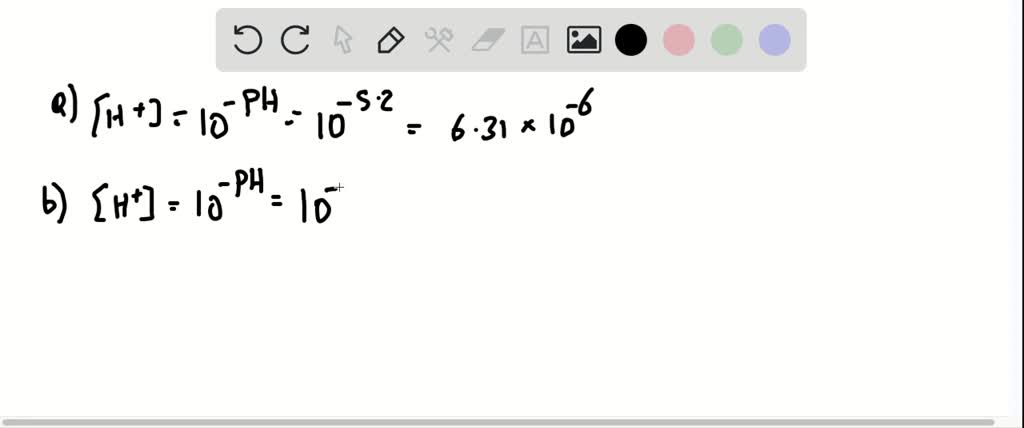
A The pH of the solution is given as 520. The hydrogen ion concentration is given by. Pure water is said to be neutral with a pH close to 70 at 25 o C 77 o F. B X concentration loss by Mg2 ion. Calculate the hydrogen ion concentration in moles per liter for a solution with a pOH of 429.
 Source: toppr.com
Source: toppr.com
The hydrogen ion concentration can be calculated using the value of Kaand the molar concentration of the weak acid. A The pH of the solution is given as 520. To solve this problem you just need to know a formula relating to pH. The pH of a solution is given by the formula pH -log H where H is the concentration of hydrogen ions in moles per liter in the solution. The hydrogen ion concentration is given by.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title calculate the hydrogen ion concentration in moles by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






