Your Calculate number of moles of element in a compound images are ready. Calculate number of moles of element in a compound are a topic that is being searched for and liked by netizens now. You can Download the Calculate number of moles of element in a compound files here. Download all free photos and vectors.
If you’re looking for calculate number of moles of element in a compound images information related to the calculate number of moles of element in a compound interest, you have visit the right site. Our website frequently provides you with suggestions for viewing the highest quality video and image content, please kindly hunt and find more enlightening video articles and graphics that match your interests.
Calculate Number Of Moles Of Element In A Compound. Explain with example. If you have a sample that contains only atoms of a particular element weigh the sample in grams and divide by the atomic weight of the element. Simply so how do you find the number of moles of an element in a compound. Number of moles formula is.
 Calculating The Moles Of An Element In A Compound Youtube From youtube.com
Calculating The Moles Of An Element In A Compound Youtube From youtube.com
NaCl 229898 gL 354530 gL. Popular Answers 1 Calculate he number of moles you have by taking the Mass molar mass. 1257g C2H6O x 1 mol C2H6O 46068g 002729 mol C2H6O. For example H2 O2 etc If the atoms constituting a molecule are different the molecule is called. Find the molar mass of sodium carbonate Na2CO3. Now to calculate its molar mass we add up all of the molar masses of each atom.
Molar Mass 5844 g m o l.
Explain with example. Find an answer to your question Calculate the number of moles of each component element in the compound. First find the number of mols in entire compound C2H6O. To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3. The number of moles formula is given by. Mass of one mole MnO2 8694g.
 Source: khanacademy.org
Source: khanacademy.org
There are nearly 118 elements at present of which nearly 94 occur naturally on Earth. For example H2 O2 etc If the atoms constituting a molecule are different the molecule is called. There are nearly 118 elements at present of which nearly 94 occur naturally on Earth. Popular Answers 1 Calculate he number of moles you have by taking the Mass molar mass. The answer is the number of moles of that mass of compound.
 Source: youtube.com
Source: youtube.com
Moles of an element in a compound calculator เผยเเพรเมอ 31 ธนวาคม 2564 โดย 0 เขาชม You can also calculate the mass of a substance needed to achieve a desired molarity. Number of moles Mass of substance Mass of one mole. Molar Mass 1000 1574. First find the number of mols in entire compound C2H6O. Moles O 545 g O x 1 mol O 1600 g O 341 mol O.
 Source: youtube.com
Source: youtube.com
M 9 12 g m o l 1 1 13 g m o l 1 14 g m o l 1 3 16 g m o l 1 183 g m o l 1. Simply so how do you find the number of moles of an element in a compound. Moles H moles S. If you have 1000 grams. For example 25 grams of water equals 2518016 or 139 moles.
 Source: studylib.net
Source: studylib.net
Second find how many molecules of each element are in it. Moles H 458 g H x 1 mol H 101 g H 453 mol H. Chemical Mole to Gram Calculator. Example If we are told the mass of each element present in a compound we can find the formula. To find the number of moles in a sample simply weigh it and divide the weight by the molecular weight.
 Source: youtube.com
Source: youtube.com
Number of moles Mass of substance Mass of one mole. This is the molar mass of the compound. Find the molar mass of sodium carbonate Na2CO3. Number of moles 95 8694. The quotient is equal.
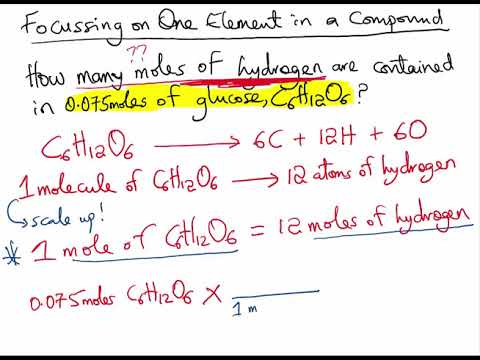 Source: youtube.com
Source: youtube.com
Molar Mass 5844 g m o l. Moles O 545 g O x 1 mol O 1600 g O 341 mol O. Now to calculate its molar mass we add up all of the molar masses of each atom. To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3. 16431022 x 2 32871022 molecules of C.
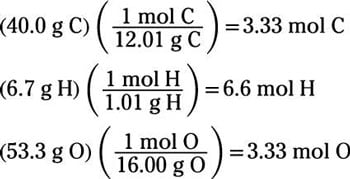 Source: dummies.com
Source: dummies.com
To find the simplest whole number ratio divide each number by the smallest number of moles. Chemical Mole to Gram Calculator. Explain with example. Molarity Calculator How do you calculate the number of moles. Simply so how do you find the number of moles of an element in a compound.
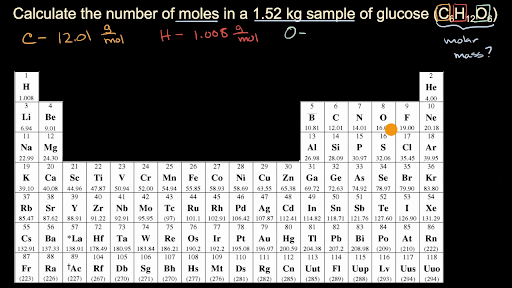
Divide the mass of the compound in grams by the molar mass you just calculated. Second find how many molecules of each element are in it. The number of moles formula is given by. This video explains how to calculate the number of moles of an element given the mass as well as how to calculate the mass given the number of moles. Example If we are told the mass of each element present in a compound we can find the formula.
 Source: toppr.com
Source: toppr.com
The answer is the number of moles of that mass of compound. Mole of a compound contains Avogadros number 6022 x 1023 of molecules molecular. Then 1000 g 151001 gmol. Number of moles formula is. Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it.
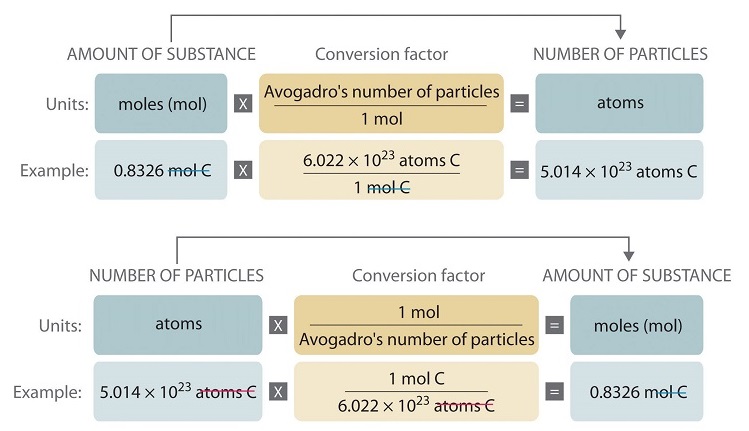 Source: chem.libretexts.org
Source: chem.libretexts.org
Molar Mass 1000 1574. There are nearly 118 elements at present of which nearly 94 occur naturally on Earth. Molarity is also considered molar concentration because it. Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it. If all the atoms constituting a molecule are same the molecule is called homo-atomic molecule.
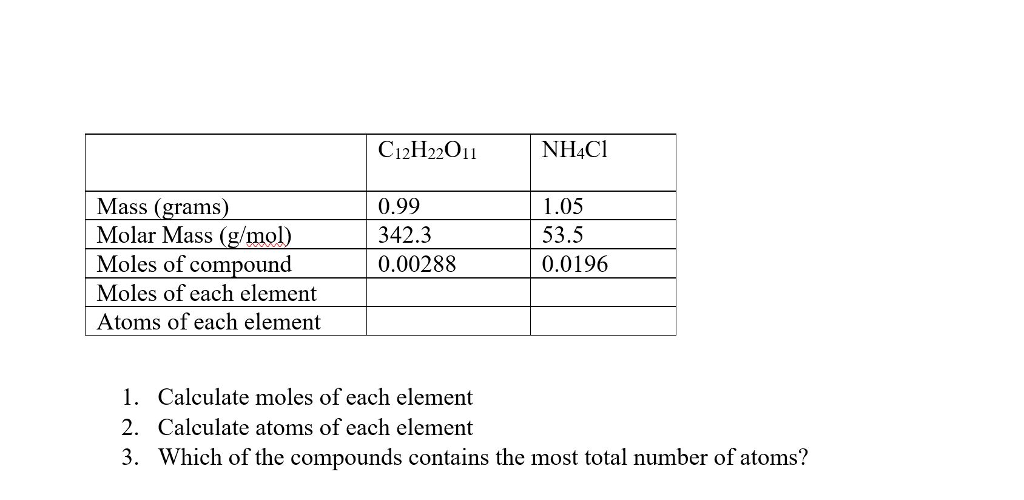 Source: chegg.com
Source: chegg.com
02729 mol C2H6O x 60221023 1 mol 16431022 molecules. Moles O 545 g O x 1 mol O 1600 g O 341 mol O. Moles of an element in a compound calculator เผยเเพรเมอ 31 ธนวาคม 2564 โดย 0 เขาชม You can also calculate the mass of a substance needed to achieve a desired molarity. One mole of any element or chemical compound is always the same number. Second find how many molecules of each element are in it.
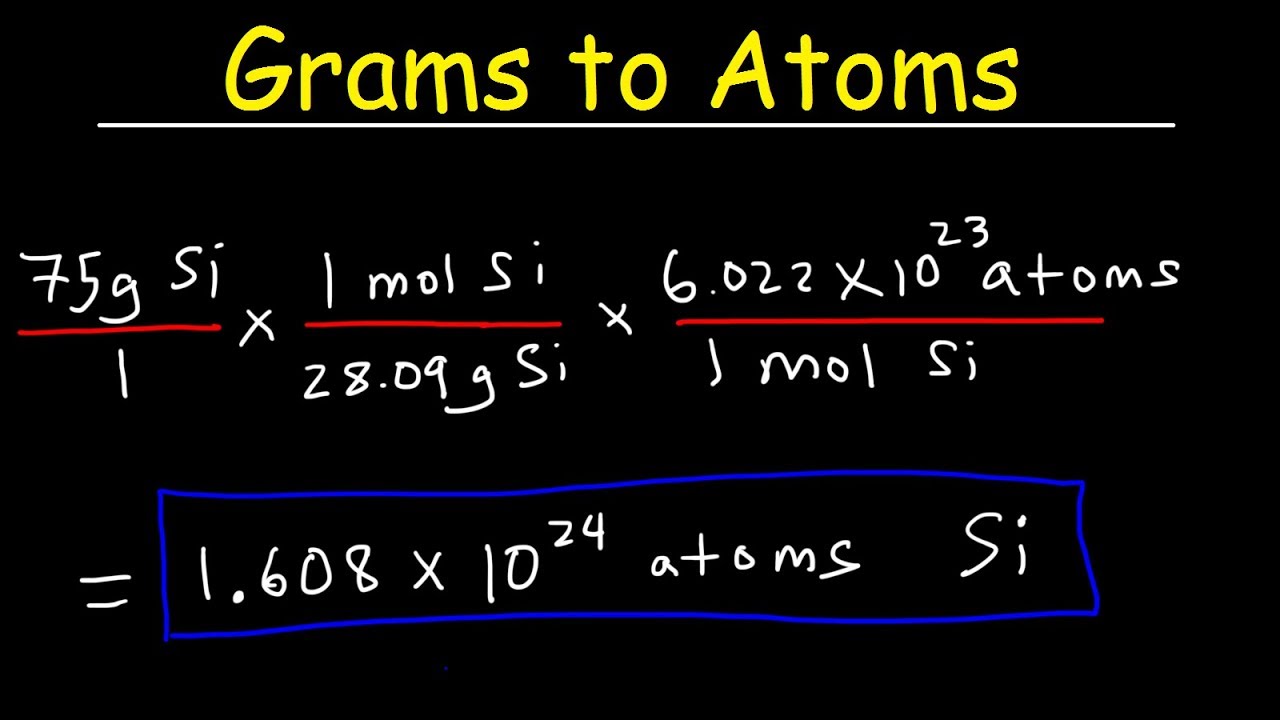 Source: youtube.com
Source: youtube.com
Moles H moles S. The quotient tells you the number of moles. 02729 mol C2H6O x 60221023 1 mol 16431022 molecules. Example If we are told the mass of each element present in a compound we can find the formula. First find the number of mols in entire compound C2H6O.
 Source: study.com
Source: study.com
Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it. Multiply that by Avogadros number and youll find out how many atoms the sample contains. This mole conversion calculator also helps you calculate molar mass of a substance using a similar mathematical approach but in less time. The quotient is equal. If you have a sample that contains only atoms of a particular element weigh the sample in grams and divide by the atomic weight of the element.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The quotient is equal. To find the number of moles in a sample simply weigh it and divide the weight by the molecular weight. Find an answer to your question Calculate the number of moles of each component element in the compound. Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it. If you have 1000 grams.
 Source: slideplayer.com
Source: slideplayer.com
Determine the number of moles in 0325g of barium hydroxide. NaCl Na Cl. Therefore NaCl 584538 gL. Number of moles 1092 mol. Mass of one mole MnO2 8694g.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title calculate number of moles of element in a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






