Your Calculate grams from molecular weight and volume images are ready in this website. Calculate grams from molecular weight and volume are a topic that is being searched for and liked by netizens now. You can Get the Calculate grams from molecular weight and volume files here. Find and Download all royalty-free photos and vectors.
If you’re looking for calculate grams from molecular weight and volume pictures information related to the calculate grams from molecular weight and volume keyword, you have come to the right site. Our website always gives you hints for seeking the maximum quality video and image content, please kindly surf and locate more enlightening video articles and graphics that match your interests.
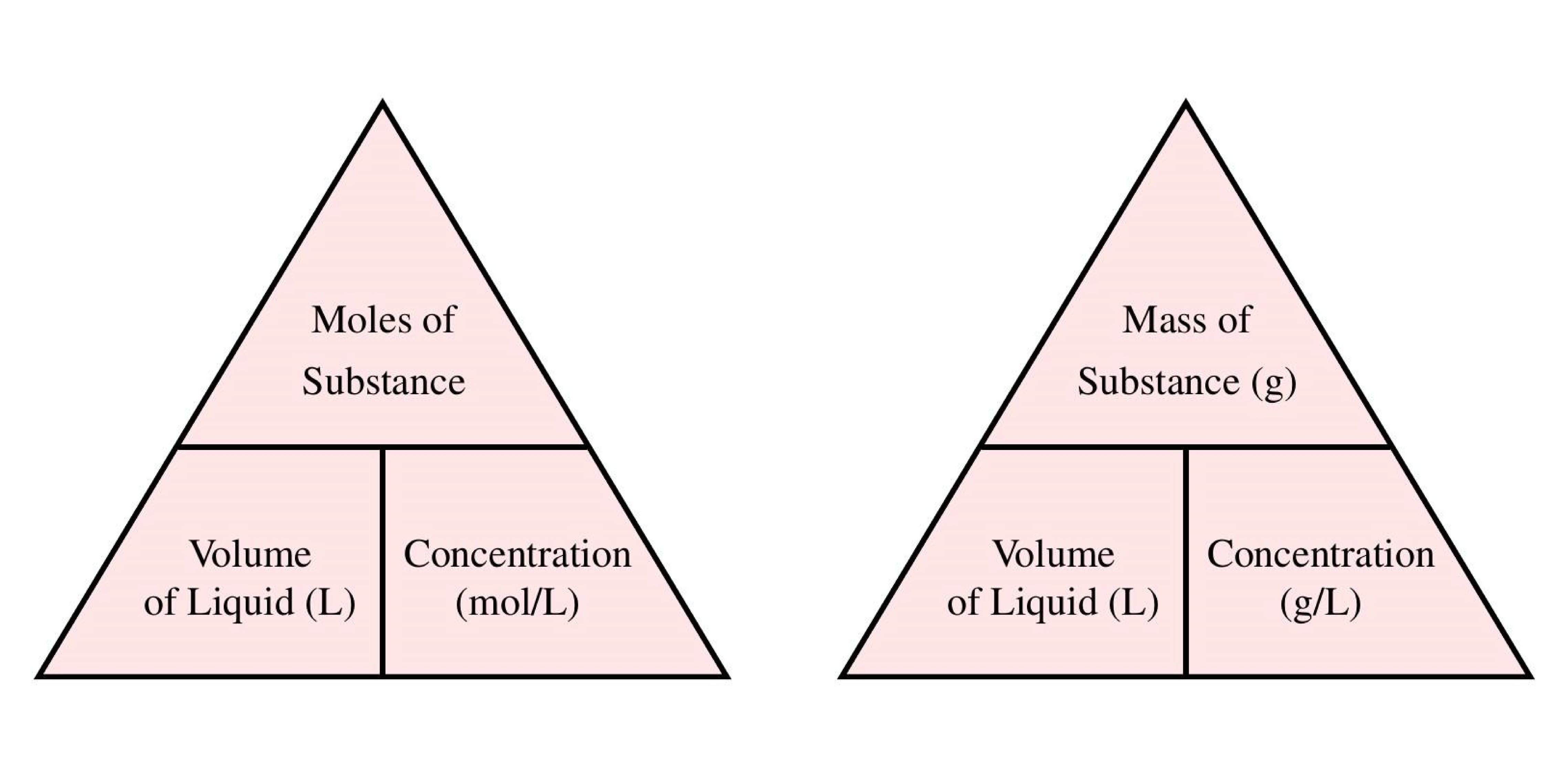
Calculate Grams From Molecular Weight And Volume. Hence mass of 224 dm 3 of a gas. Meant to be used in both the teaching and research laboratory this calculator see below can be utilized to perform a number of different calculations for preparing solutions having mass per volume ie mass over volume or weight per volume ie weight over volume concentration units such as mgmL μgμL μgL etc. Then we divide the number of moles by the total solution volume to get concentration. Volume and weight of the substance is calculated using its molecular weight density and number of moles.
 Concentration Expression 1 Percentage Weightinvolume L The Grams From slidetodoc.com
Concentration Expression 1 Percentage Weightinvolume L The Grams From slidetodoc.com
Hence mass of 224 dm 3 of a gas. Then we divide the number of moles by the total solution volume to get concentration. The mass of the object divided by its volume. Notice volume of solution is in liters. Weight per weight ww. Most of your household chemicals are solutions.
Ml of substance in 100 ml of solution volume of solutevolume of solution 100.
Molecular mass 179759125 x 224 44. Most of your household chemicals are solutions. Meant to be used in both the teaching and research laboratory this calculator see below can be utilized to perform a number of different calculations for preparing solutions having mass per volume ie mass over volume or weight per volume ie weight over volume concentration units such as mgmL μgμL μgL etc. There are two steps. Ml of substance in 100 ml of solution volume of solutevolume of solution 100. Divide the mass by the molar mass to get the number of moles.
 Source: khanacademy.org
Source: khanacademy.org
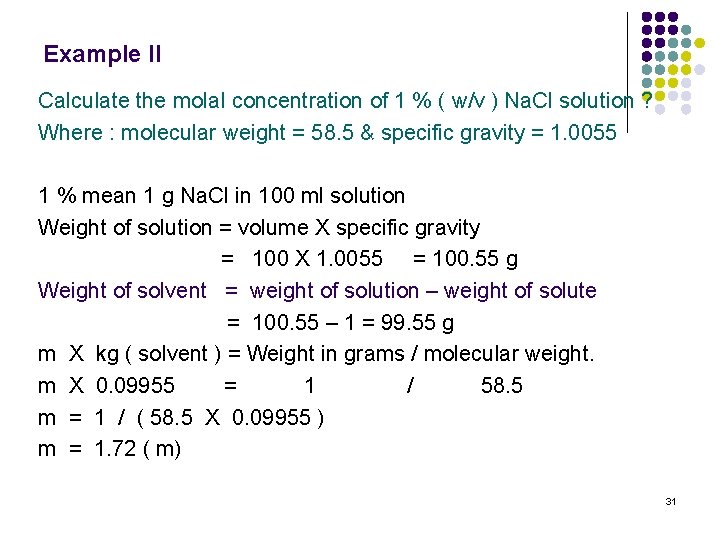
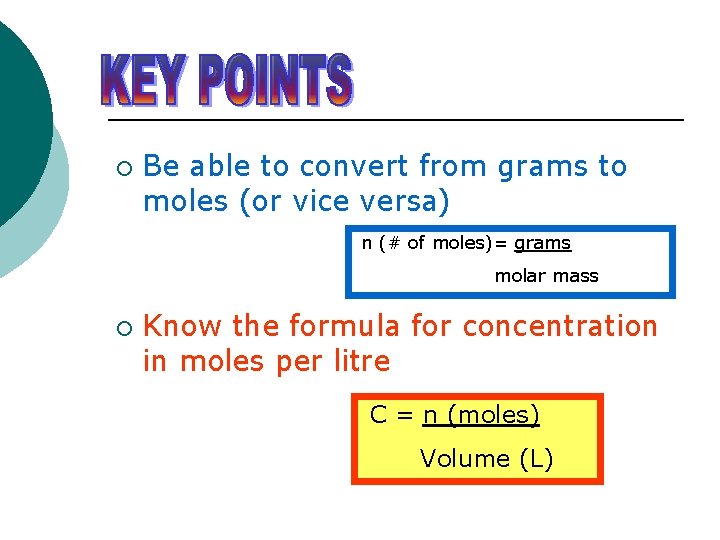
In ml 100 volume per volume vv. R 0082 057 33847 L atm K1 mol1 that is a relative standard uncertainty of 57107 according to the 2014. C nv 01 n05 500 ml 05 dm 3 n 005mol. Molarity is molesliter. This can be converted to grams by multiplying the number of moles by the molar mass of the solute.
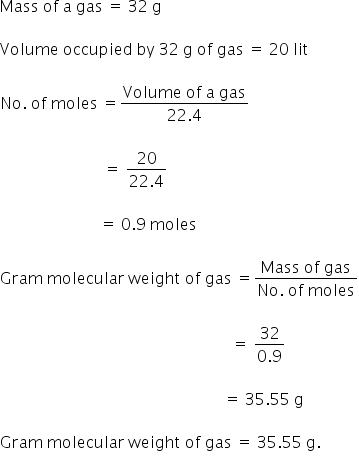 Source: topperlearning.com
Source: topperlearning.com
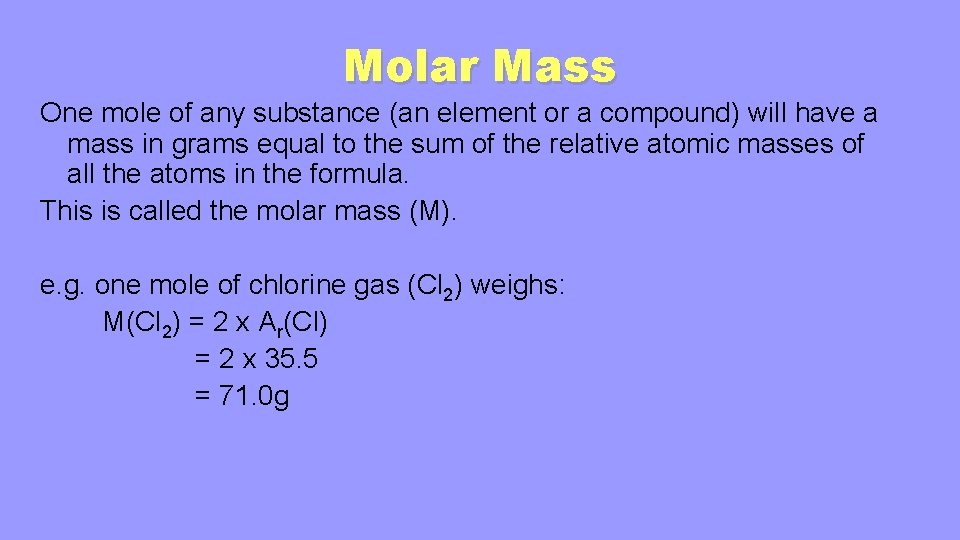
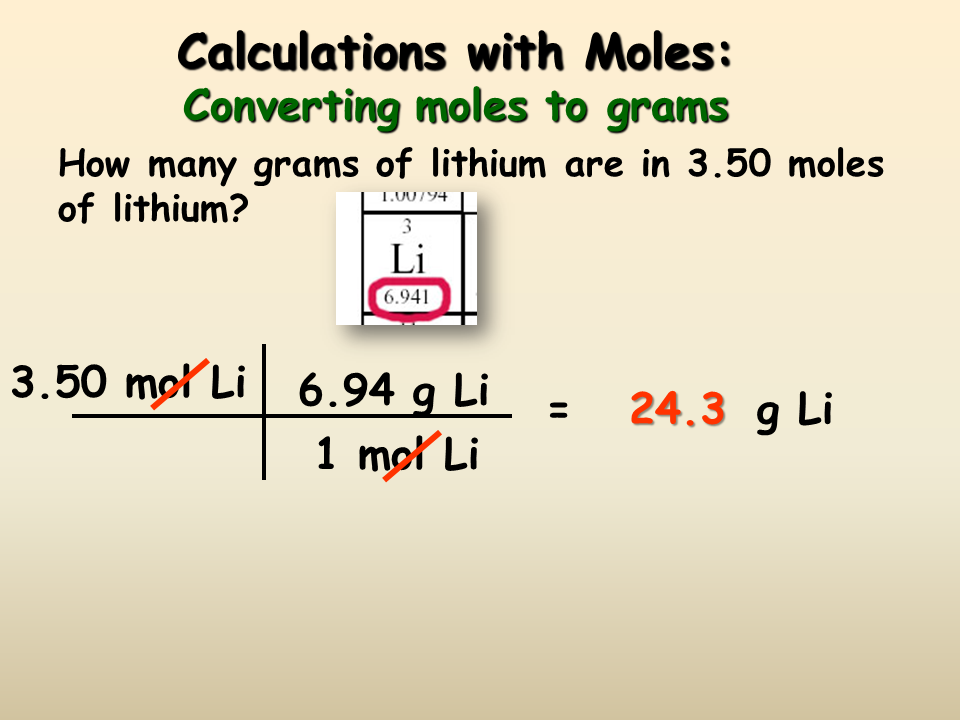
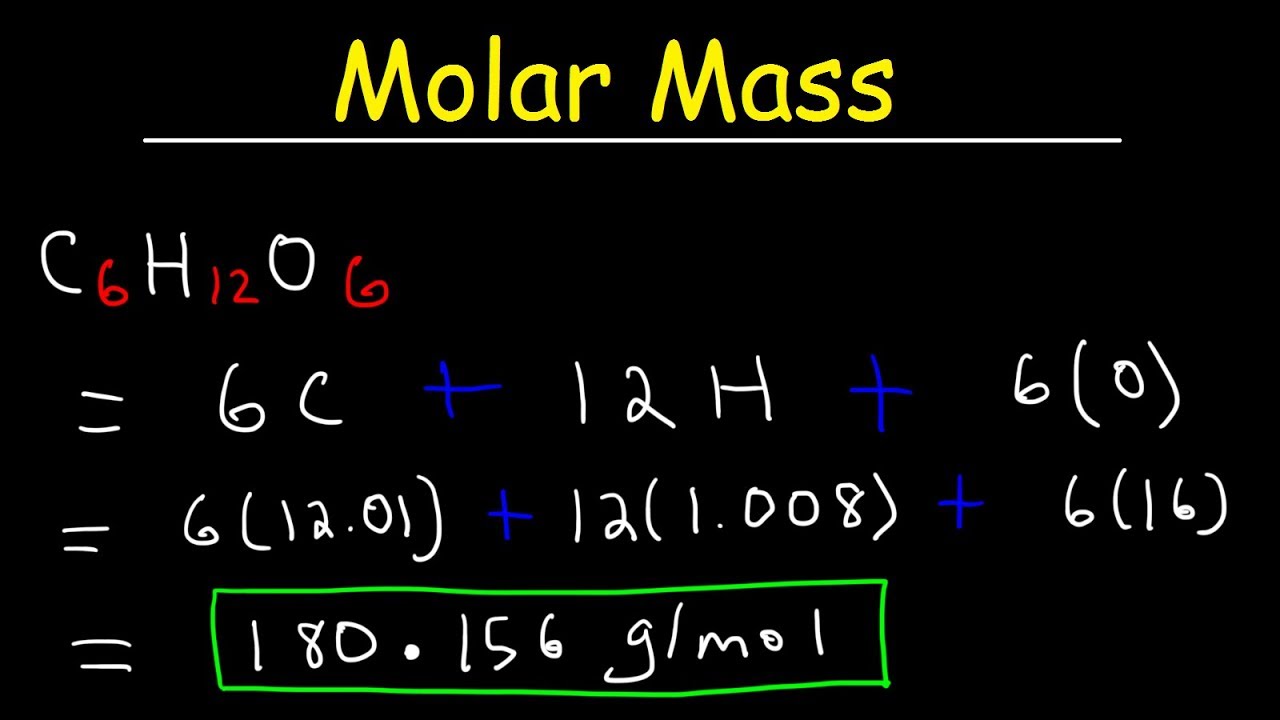
In your everyday life you encounter solutionsall the time. A list of approximations is available. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. From molesliter you can calculate mass in grams by converting the moles to grams using molar mass and then use the volume to calculate the final mass in that volume. Mass g Concentration molL x Volume L x Molecular Weight gmol An example of a molarity calculation using the Tocris molarity calculator.
 Source: topperlearning.com
Source: topperlearning.com
Notice volume of solution is in liters. Mass g Concentration molL x Volume L x Molecular Weight gmol An example of a molarity calculation using the Tocris molarity calculator. Multiply the volume by the density to get the mass. The second problem will ask you to determine the mass molarity or volume given the two that are given and the molecular weight using variations of. Weight per weight ww.
 Source: ncl.ac.uk
Source: ncl.ac.uk
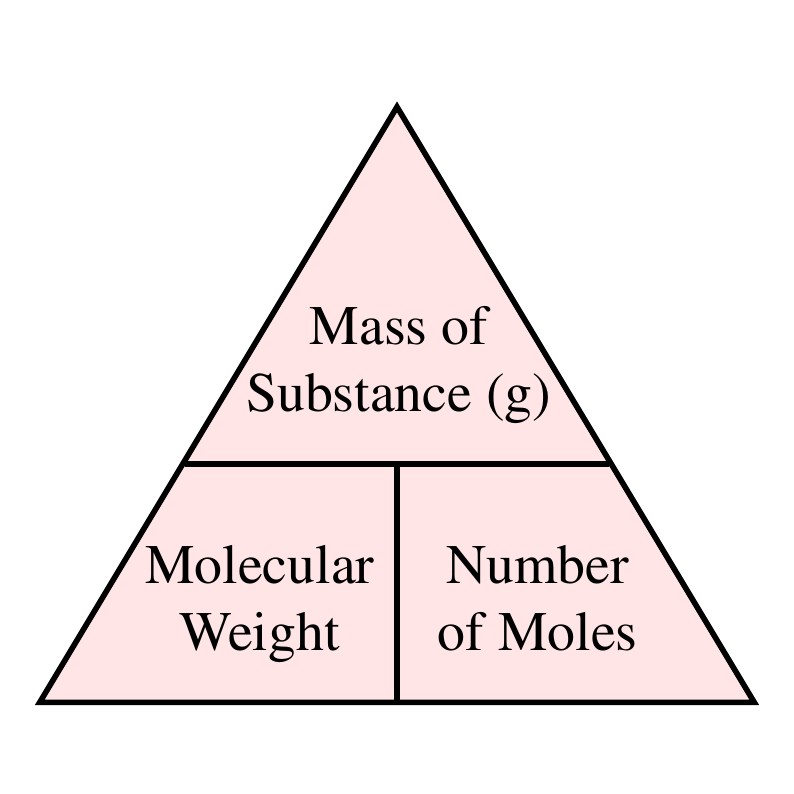
M mass n moles M molecular weight n mM m molecular weight moles m Mn m 005 40 m 2g. Hence mass of 224 dm 3 of a gas. Typical unit is gmol. For example you may add salt to water when cooking pasta. V o 5 litres 5 dm 3 W 144 g.
 Source: slidetodoc.com
Source: slidetodoc.com
Please enter a density figure select a unit to. NaOH amount 005 mol. In your everyday life you encounter solutionsall the time. If you know the volume of the solution in liters you can multiply the molarity by the volume in liters to determine the number of moles of solute. A list of approximations is available.
 Source: slideplayer.com
Source: slideplayer.com
A list of approximations is available. Notice volume of solution is in liters. Volume L Mass g Concentration molL x Molecular Weight gmol molecular weight of a solvent in a solution calculation. As explained in the article how to convert from volume to weight to convert between weight and volume accurately you need to know the density of the substance that you are trying to convert. The ideal gas equation is a good approximation for many common gases.
 Source: thefactfactor.com
Source: thefactfactor.com
From molesliter you can calculate mass in grams by converting the moles to grams using molar mass and then use the volume to calculate the final mass in that volume. Then we divide the number of moles by the total solution volume to get concentration. Ml of substance in 100 ml of solution volume of solutevolume of solution 100. Multiply the volume by the density to get the mass. Mass g Concentration molL x Volume L x Molecular Weight gmol volume of a solution calculation.
 Source: slidetodoc.com
Source: slidetodoc.com
Mass g Concentration molL x Volume L x Molecular Weight gmol An example of a molarity calculation using the Tocris molarity calculator. Such concentration calculations are. Most of your household chemicals are solutions. V o 5 litres 5 dm 3 W 144 g. Volume L Mass g Concentration molL x Molecular Weight gmol molecular weight of a solvent in a solution calculation.
 Source: slidetodoc.com
Source: slidetodoc.com
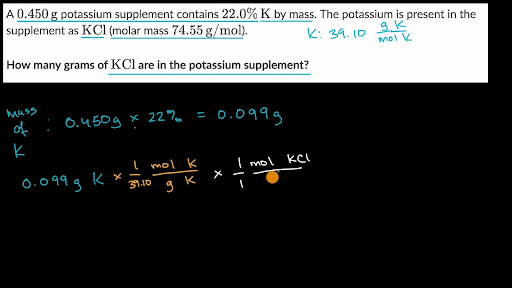
V o 5 litres 5 dm 3 W 144 g. The second problem will ask you to determine the mass molarity or volume given the two that are given and the molecular weight using variations of. Volume and weight of the substance is calculated using its molecular weight density and number of moles. What is the mass of compound required to make a 10 mM stock solution in 10 ml of water given that the molecular weight of the compound is 19713 gmol. As explained in the article how to convert from volume to weight to convert between weight and volume accurately you need to know the density of the substance that you are trying to convert.
Concentration moles amount and volume relation is used. What is the mass of compound required to make a 10 mM stock solution in 10 ml of water given that the molecular weight of the compound is 19713 gmol. 156 g 00371245 mol 42020768 gmol. We do this by dividing by the molecular weight of NaCl 584 gmole. In your everyday life you encounter solutionsall the time.
 Source: ncl.ac.uk
Source: ncl.ac.uk
Volume and weight of the substance is calculated using its molecular weight density and number of moles. 156 g 00371245 mol 42020768 gmol. C nv 01 n05 500 ml 05 dm 3 n 005mol. In the first problem practice determining either moles volume or molarity from the two that are given using variations of equation 1. NaOH amount 005 mol.
 Source: youtube.com
Source: youtube.com
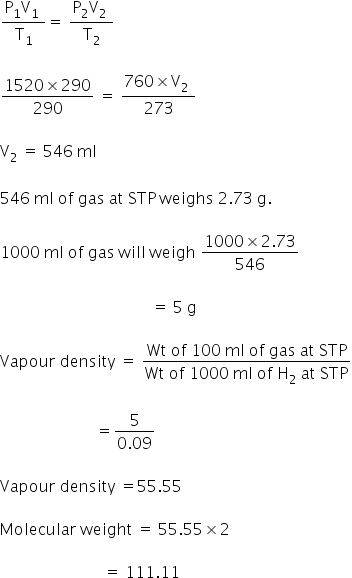
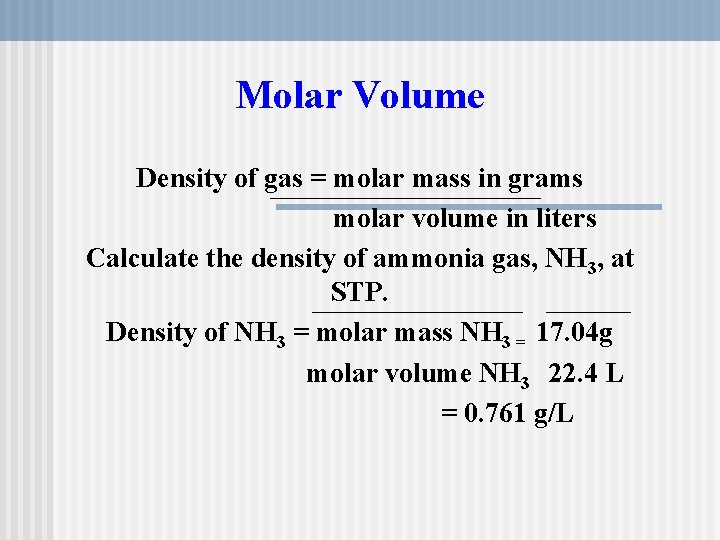
As explained in the article how to convert from volume to weight to convert between weight and volume accurately you need to know the density of the substance that you are trying to convert. Typical units are gmL and kgm3. The mass of the object divided by its volume. The ideal gas equation is a good approximation for many common gases. The relative molecular mass of the gas is 44 g.
This can be converted to grams by multiplying the number of moles by the molar mass of the solute. Molarity is molesliter. The mass of the object divided by its volume. Find the required weightg Multiply required moles times molecular weight. In the first problem practice determining either moles volume or molarity from the two that are given using variations of equation 1.
 Source: slideplayer.com
Source: slideplayer.com
N PVRT n 0984 atm1 L008206 L atm mol-1 K-1 323 K n 00371245 mol. This can be converted to grams by multiplying the number of moles by the molar mass of the solute. Mass g Concentration molL x Volume L x Molecular Weight gmol An example of a molarity calculation using the Tocris molarity calculator. Concentration moles amount and volume relation is used. Grams of substance in 100 ml of solution mass of solute in gvolume of sol.
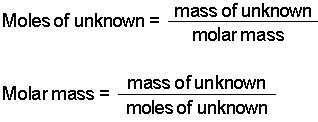 Source: chem.purdue.edu
Source: chem.purdue.edu
In your everyday life you encounter solutionsall the time. This property simplifies many chemical computations. Meant to be used in both the teaching and research laboratory this calculator see below can be utilized to perform a number of different calculations for preparing solutions having mass per volume ie mass over volume or weight per volume ie weight over volume concentration units such as mgmL μgμL μgL etc. The salt dissolves in the water resulting in a solution. Find the required weightg Multiply required moles times molecular weight.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title calculate grams from molecular weight and volume by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.