Your B how did you convert grams to moles images are ready. B how did you convert grams to moles are a topic that is being searched for and liked by netizens now. You can Get the B how did you convert grams to moles files here. Download all royalty-free photos and vectors.
If you’re looking for b how did you convert grams to moles images information linked to the b how did you convert grams to moles interest, you have visit the right site. Our website frequently gives you hints for seeing the maximum quality video and picture content, please kindly surf and locate more informative video content and images that match your interests.
B How Did You Convert Grams To Moles. Use this page to learn how to. Divide the number of grams of the substance by the molecular mass. Enter the moles and formula weight in the input field. How do you convert grams to molecules.
 Stoichiometry Quiz Conversions To Moles Grams Etc In 2021 Teaching Chemistry Quiz Chemistry From gr.pinterest.com
Stoichiometry Quiz Conversions To Moles Grams Etc In 2021 Teaching Chemistry Quiz Chemistry From gr.pinterest.com
Divide the number of grams of the substance by the molecular mass. Use this page to learn how to. Enter the moles and formula weight in the input field. Limiting Reagent Examples Solution. How many atoms do you think this amount represents. 1 Convert everything into moles by dividing each 500 g by their respective molar masses.
9 moles co2 to grams 3960855 grams.
How many atoms are there. Then add all your answers together to find the molar mass of the compound. How do you convert grams to moles examples. You convert the grams to moles by dividing the grams to moles. 1 grams Boron 0092498381278328 mole using the molecular weight calculator and the molar mass of B. To convert among any units in the left column say from A to B you can multiply by the factor for A to convert A into grams then divide by.
 Source: youtube.com
Source: youtube.com
How did you convert grams to moles. Divide the number of grams of the substance by the molecular mass. We can use this to create the conversion factors 1 mol H_2O1802 g H_2O and 1802 g H_2O1 mol H_2O. So if you have the chemical formula you add up the atomic weights of each element multiplying by the occurrances of that element in the formula and the total gives grams per mole. Start by multiplying the number of atoms by the atomic weight for each element in the compound.
 Source: pinterest.com
Source: pinterest.com
Then add all your answers together to find the molar mass of the compound. Na 2 B 4 O 7 — 002485 mol H 2 SO 4 — 005097 mol H 2 O — 02775 mol 2 Note that there are three reactants. 1 grams Methane is equal to 0062334579609362 mole. Pour the jars into the atom counter at left. 1 Convert everything into moles by dividing each 500 g by their respective molar masses.
 Source: pinterest.com
Source: pinterest.com
Use this page to learn how to. This will give you the molecular mass of the molecule. First of all find the number of moles by using mass and molar mass. So if you have the chemical formula you add up the atomic weights of each element multiplying by the occurrances of that element in the formula and the total gives grams per mole. The adult dosage of elixophyllin is 6 mgkg.
 Source: pinterest.com
Source: pinterest.com
You convert the grams to moles by dividing the grams to moles. 1 grams Boron 0092498381278328 mole using the molecular weight calculator and the molar mass of B. Start with 2000 moles of sulfur then press Start A. How do you convert grams to molecules. Divide the number of grams of the substance by the molecular mass.
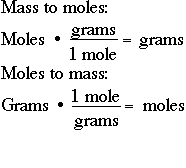 Source: socratic.org
Source: socratic.org
Enter the moles and formula weight in the input field. We know that 1 mol H₂O 1802 g H₂O. How many atoms are there. How did you convert grams to moles. I think the atoms this amount represents is 1204 x 1024 B.
 Source: pinterest.com
Source: pinterest.com
This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions. After doing so multiply the moles with the Avogadros number. To convert from g into units in the left column divide by the value in the right column or multiply by the reciprocal 1x. How do you convert grams to moles examples. The answer is the number of moles of that mass of compoundFor example 25 grams of water equals 2518016 or 139 moles.
 Source: wikihow.com
Source: wikihow.com
How many atoms do you think this amount represents. This will give you the molecular mass of the molecule. To convert from g into units in the left column divide by the value in the right column or multiply by the reciprocal 1x. First of all find the number of moles by using mass and molar mass. Enter the moles and formula weight in the input field.
 Source: pinterest.com
Source: pinterest.com
You can convert the grams to molecules by following the steps below. First convert the molecules into moles of carbon dioxide question_answer Q. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. The adult dosage of elixophyllin is 6 mgkg. Pour the jars into the atom counter at left.
 Source: youtube.com
Source: youtube.com
Start by multiplying the number of atoms by the atomic weight for each element in the compound. Convert 250 g of water to moles of water. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions. Use this page to learn how to. The answer is the number of moles of that mass of compoundFor example 25 grams of water equals 2518016 or 139 moles.
 Source: pinterest.com
Source: pinterest.com
Start with 2000 moles of sulfur then press Start A. We know that 1 mol H₂O 1802 g H₂O. Calculate the dose in milligrams for a 150lb person. How do you convert grams to moles examples. How did you convert grams to moles.
 Source: pinterest.com
Source: pinterest.com
After doing so multiply the moles with the Avogadros number. 1 grams Boron 0092498381278328 mole using the molecular weight calculator and the molar mass of B. So if you have the chemical formula you add up the atomic weights of each element multiplying by the occurrances of that element in the formula and the total gives grams per mole. To convert from g into units in the left column divide by the value in the right column or multiply by the reciprocal 1x. How is the limiting reagent determined when there are three.
 Source: gr.pinterest.com
Source: gr.pinterest.com
Divide the mass of the compound in grams by the molar mass you just calculated. We can use this to create the conversion factors 1 mol H_2O1802 g H_2O and 1802 g H_2O1 mol H_2O. First of all find the number of moles by using mass and molar mass. Start with 2000 moles of sulfur then press Start. The atomic weight is the number of grams of that element that make a mole.
 Source: gr.pinterest.com
Source: gr.pinterest.com
How many atoms are there. You can do the reverse unit conversion from grams ga to moles or enter other units to convert below. Limiting Reagent Examples Solution. Do a quick conversion. Note that rounding errors may occur so always check the results.
 Source: wikihow.com
Source: wikihow.com
Multiply the moles by the molar mass. Multiply the moles by the molar mass. 1 Convert everything into moles by dividing each 500 g by their respective molar masses. This will give you the molecular mass of the molecule. Use this page to learn how to.
 Source: no.pinterest.com
Source: no.pinterest.com
Then add all your answers together to find the molar mass of the compound. Rock Chalk Jayhawk KU. Activity B continued on next page Activity B continued from previous page 4. So if you have the chemical formula you add up the atomic weights of each element multiplying by the occurrances of that element in the formula and the total gives grams per mole. Use this page to learn how to.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title b how did you convert grams to moles by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






