Your Average mass of one mole of oxygen images are ready. Average mass of one mole of oxygen are a topic that is being searched for and liked by netizens now. You can Download the Average mass of one mole of oxygen files here. Get all royalty-free photos and vectors.
If you’re searching for average mass of one mole of oxygen images information connected with to the average mass of one mole of oxygen topic, you have pay a visit to the ideal blog. Our site always gives you suggestions for viewing the maximum quality video and picture content, please kindly hunt and locate more enlightening video content and images that match your interests.
Average Mass Of One Mole Of Oxygen. 7 moles Oxygen to grams 1119958 grams. 115 rows One molecule of water H 2 O would weigh 1802 amu 2100797 amu for H. Assume air has an average molar mass of 28 gramsmole and determine how many moles of air molecules there are 10 liters of air which contains 126 grams of air molecules. Oxygen has a molar mass of 159994 gmol and nitrogen has a molar mass of 140067 gmol.
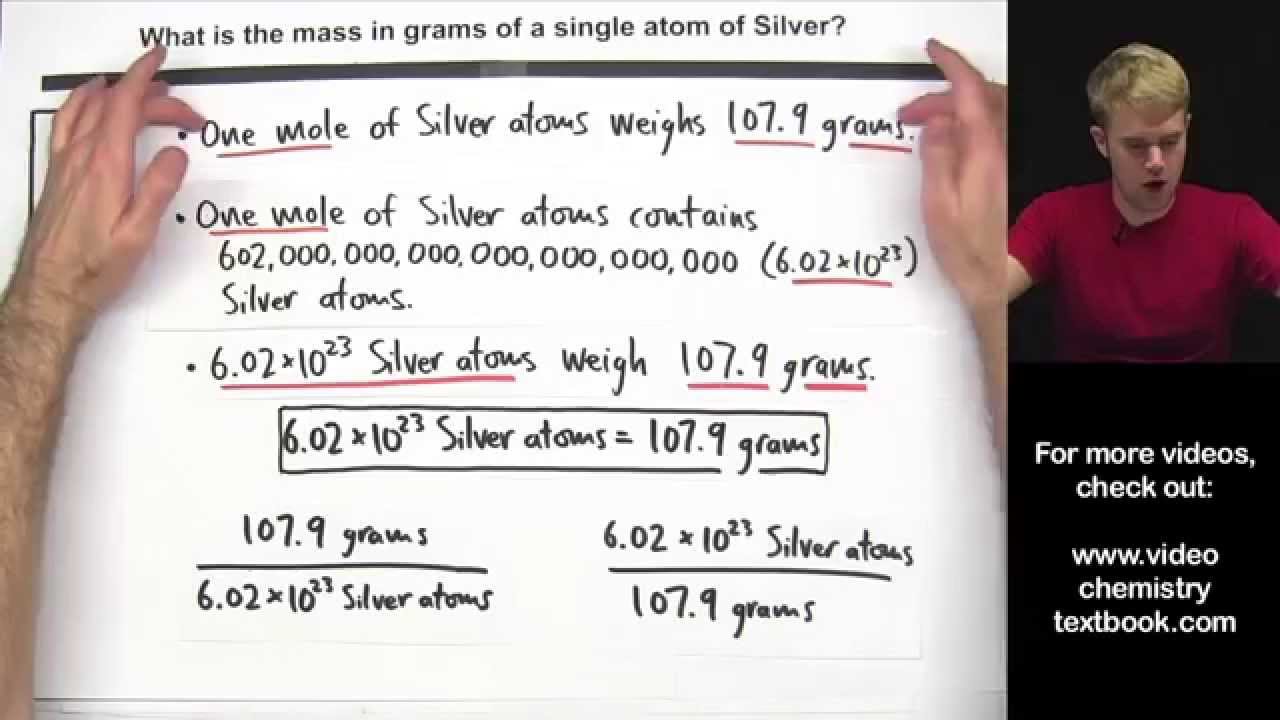 Chemteam Average Mass Of One Molecule From chemteam.info
Chemteam Average Mass Of One Molecule From chemteam.info
B Almost all O atoms are 18 O. In Imperial or US customary measurement system the density is equal to 008921 pound per cubic foot lbft³. A Mass of one mole of oxygen atoms 16 g. When we say the molar mass of carbon is 120 g mol 1 it means one mole of carbon weighs 1201 g. This was calculated by multiplying the atomic weight of hydrogen 1008 by two and adding the result to the weight for one oxygen 15999. Please remember that you need the molar mass first when trying.
For example one mole of oxygen is 15999 grams one mole of hydrogen has a mass of 101 grams one mole of carbon has a mass of 1202 grams and so on.
In Imperial or US customary measurement system the density is equal to 008921 pound per cubic foot lbft³. 3 moles Oxygen to grams 479982 grams. 101 grams of hydrogen is greater than 101 amu of hydrogen. 8 moles Oxygen to grams 1279952 grams. A mole of a substance is referred to the mass of a substance containing the same number of fundamental units as there are atoms in exactly 12000 g of 12C. At 0C 32F or 27315K at standard atmospheric pressure.
 Source: youtube.com
Source: youtube.com
Due to the use of the same reference substance in defining the atomic mass unit and the mole the formula mass amu and molar mass gmol for any substance are numerically equivalent for example one H 2 O molecule weighs approximately18 amu and 1 mole of H 2 O molecules weighs approximately 18 g. 4 moles Oxygen to grams 639976 grams. 115 rows One molecule of water H 2 O would weigh 1802 amu 2100797 amu for H. 2 moles Oxygen to grams 319988 grams. 3 moles Oxygen to grams 479982 grams.

Molar mass of oxygen 162gm 32gm. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms. A mole of a substance is referred to the mass of a substance containing the same number of fundamental units as there are atoms in exactly 12000 g of 12C. 32 gmol and the molar mass of nitrogen gas is aprox. What can be implied about the relative abundances of these isotopes.
 Source: youtube.com
Source: youtube.com
Amole of nitrogen atoms is 14 grams and a mole of nitrogen molecules 28 gramsetc. At 0C 32F or 27315K at standard atmospheric pressure. Oxygen has a molar mass of 159994 gmol and nitrogen has a molar mass of 140067 gmol. Thus mass of 1 mole of oxygen is 32gm. B Mass of one mole of water molecule 18 g.

7 moles Oxygen to grams 1119958 grams. 3H 3100 300 amu Sum 17 amu Molar mass is 17 gmol Or one mole would be 17g. B Almost all O atoms are 18 O. C Almost all O atoms are 17 O. Due to the use of the same reference substance in defining the atomic mass unit and the mole the formula mass amu and molar mass gmol for any substance are numerically equivalent for example one H 2 O molecule weighs approximately18 amu and 1 mole of H 2 O molecules weighs approximately 18 g.

115 rows One molecule of water H 2 O would weigh 1802 amu 2100797 amu for H. A mole of oxygen atoms 16 grams while a mole of oxygen molecules is 32 grams. 1 moles Oxygen to grams 159994 grams. Due to the use of the same reference substance in defining the atomic mass unit and the mole the formula mass amu and molar mass gmol for any substance are numerically equivalent for example one H 2 O molecule weighs approximately18 amu and 1 mole of H 2 O molecules weighs approximately 18 g. A mole of a substance is referred to the mass of a substance containing the same number of fundamental units as there are atoms in exactly 12000 g of 12C.
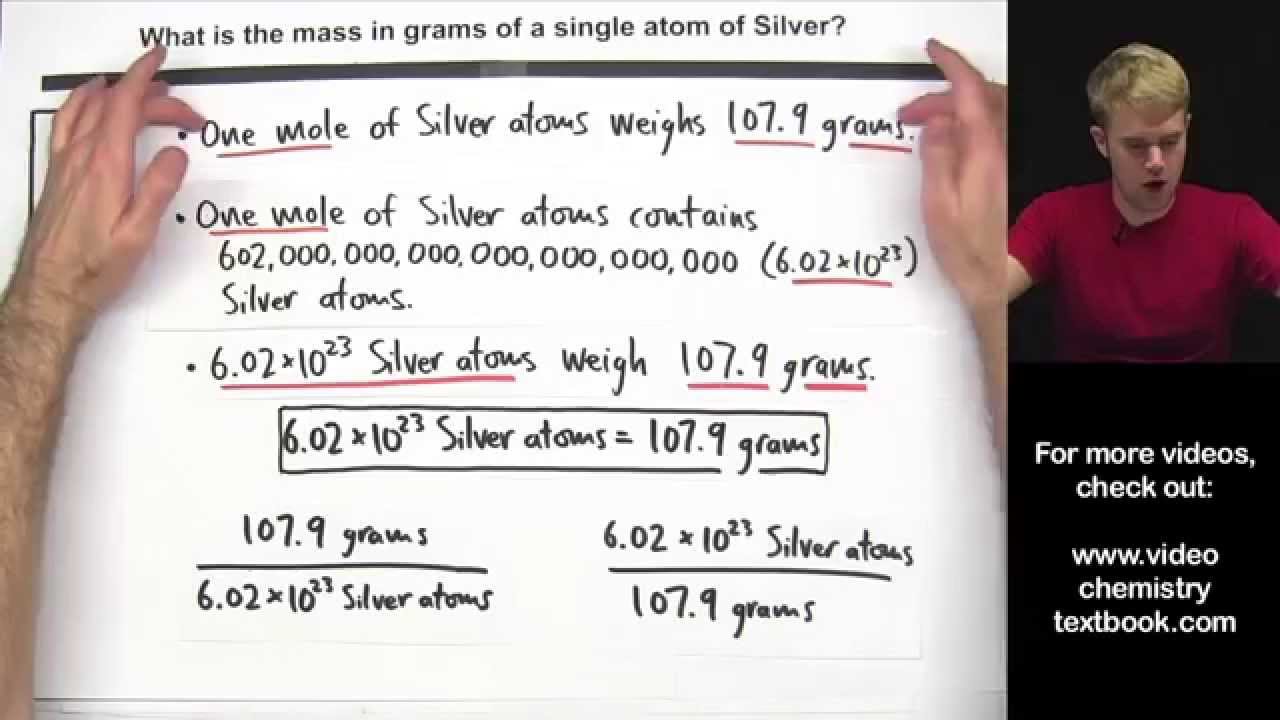 Source: chemteam.info
Source: chemteam.info
Relating atomic weights to relative isotope-ratio measurements of oxygen may be complicated in principleby the observation that the exponent in the mass-dependent fractionation equation may deviate significantlyfrom one half and by the fact that relative isotope-ratio. In other words 6022 10 23 atoms of carbon weigh 1201 g. Relating atomic weights to relative isotope-ratio measurements of oxygen may be complicated in principleby the observation that the exponent in the mass-dependent fractionation equation may deviate significantlyfrom one half and by the fact that relative isotope-ratio. 8 moles Oxygen to grams 1279952 grams. Oxygen has a molar mass of 159994 gmol and nitrogen has a molar mass of 140067 gmol.
 Source: youtube.com
Source: youtube.com
Due to the use of the same reference substance in defining the atomic mass unit and the mole the formula mass amu and molar mass gmol for any substance are numerically equivalent for example one H 2 O molecule weighs approximately18 amu and 1 mole of H 2 O molecules weighs approximately 18 g. A mole of a substance is referred to the mass of a substance containing the same number of fundamental units as there are atoms in exactly 12000 g of 12C. In other words 6022 10 23 atoms of carbon weigh 1201 g. 6 moles Oxygen to grams 959964 grams. What can be implied about the relative abundances of these isotopes.

Assume air has an average molar mass of 28 gramsmole and determine how many moles of air molecules there are 10 liters of air which contains 126 grams of air molecules. What is the mass of one mole of. In other words 6022 10 23 atoms of carbon weigh 1201 g. A mole of a substance is referred to the mass of a substance containing the same number of fundamental units as there are atoms in exactly 12000 g of 12C. For atoms the atomic mass of an element corresponds to the average mass of a single atom in amu And The mass of a mole of atoms in grams.
 Source: youtube.com
Source: youtube.com
Convert grams Oxygen to moles or moles Oxygen to grams Percent composition by element. For example one mole of oxygen is 15999 grams one mole of hydrogen has a mass of 101 grams one mole of carbon has a mass of 1202 grams and so on. 101 amu of hydrogen or 101 grams of hydrogen. A mole of oxygen atoms 16 grams while a mole of oxygen molecules is 32 grams. What can be implied about the relative abundances of these isotopes.
 Source: toppr.com
Source: toppr.com
In other words 6022 10 23 atoms of carbon weigh 1201 g. 8 moles Oxygen to grams 1279952 grams. At 0C 32F or 27315K at standard atmospheric pressure. 101 grams of hydrogen is greater than 101 amu of hydrogen. Amole of nitrogen atoms is 14 grams and a mole of nitrogen molecules 28 gramsetc.
 Source: slideplayer.com
Source: slideplayer.com
8 moles Oxygen to grams 1279952 grams. The element oxygen consists of three naturally occuring isotopes. Assume air has an average molar mass of 28 gramsmole and determine how many moles of air molecules there are 10 liters of air which contains 126 grams of air molecules. In Imperial or US customary measurement system the density is equal to 008921 pound per cubic foot lbft³. 1 10 -6.
 Source: youtube.com
Source: youtube.com
32 gmol and the molar mass of nitrogen gas is aprox. Relating atomic weights to relative isotope-ratio measurements of oxygen may be complicated in principleby the observation that the exponent in the mass-dependent fractionation equation may deviate significantlyfrom one half and by the fact that relative isotope-ratio. 2 moles Oxygen to grams 319988 grams. The atomic mass of oxygen is 1600 amu. What can be implied about the relative abundances of these isotopes.

4 moles Oxygen to grams 639976 grams. Oxygen has a molar mass of 159994 gmol and nitrogen has a molar mass of 140067 gmol. Oxygen has a molar mass of 159994 gmol and nitrogen has a molar mass of 140067 gmol. Then the mass of 02 mole of oxygen atoms 02 x 16g 32 g. 2 moles Oxygen to grams 319988 grams.
 Source: youtube.com
Source: youtube.com
Molar mass of oxygen 162gm 32gm. Molecular Mass of NO2Weight of Nitrogen 14Weight of Oxygen 162 32Molecular mass 143246. The atomic mass of oxygen is 160 amu. A Mass of one mole of oxygen atoms 16 g. 5 moles Oxygen to grams 79997 grams.

A mole of a substance is referred to the mass of a substance containing the same number of fundamental units as there are atoms in exactly 12000 g of 12C. Please remember that you need the molar mass first when trying. Since both of these elements are diatomic in air - O 2 and N 2 the molar mass of oxygen gas is aprox. Since both of these elements are diatomic in air - O2 and N2 the molar mass of oxygen gas is 32 gmol and the molar mass of nitrogen gas is 28 gmol. Amole of nitrogen atoms is 14 grams and a mole of nitrogen molecules 28 gramsetc.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title average mass of one mole of oxygen by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






