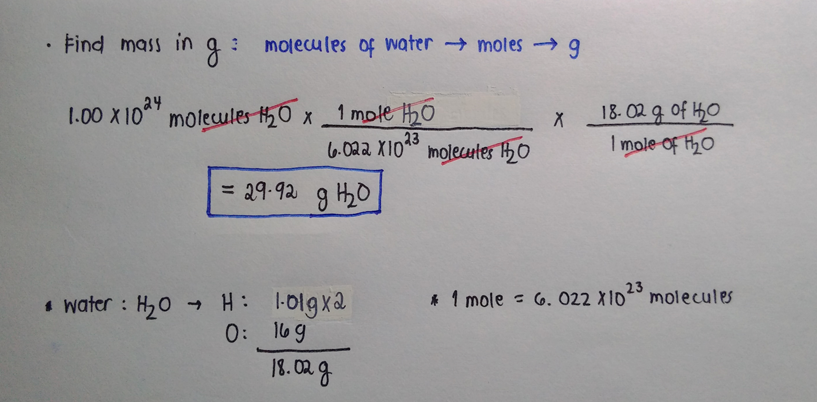Your Atomic weight of iron in grams per mole images are ready. Atomic weight of iron in grams per mole are a topic that is being searched for and liked by netizens today. You can Find and Download the Atomic weight of iron in grams per mole files here. Get all royalty-free images.
If you’re looking for atomic weight of iron in grams per mole images information connected with to the atomic weight of iron in grams per mole interest, you have pay a visit to the right blog. Our website always provides you with hints for seeking the maximum quality video and picture content, please kindly hunt and find more enlightening video content and graphics that match your interests.
Atomic Weight Of Iron In Grams Per Mole. Molar mass of Fe is 56 g mol-1 1 mole of iron weights 56 grams 1 mole of Fe contain 6022 x 10 23 atoms therefore 74 mole of Fe contain 74 x 6022 x 10 23 atoms 446 x 10 23 atoms 446 x 10 24 atoms 74 mole of iron contains 446 x. It dissolves readily in dilute acids. Density of iron is equal to 7 873 kgm³. The definition of atomic mass the mole and molar mass are all directly or indirectly related to carbon-12.

For example iron Fe has a gram atomic mass of 55845 g due to its atomic weight of 55845 u. M molar mass gmol 55845 gmol Fe atomic weight from periodic table in gramsmole n 10 g Fe55845 gmol 0179 mol Fe. This leads to two important facts. 1 grams Iron 001790670606142 mole using the molecular weight calculator and the molar mass of Fe. More information from the unit converter. Molar mass massmole gmol.
M molar mass gmol 55845 gmol Fe atomic weight from periodic table in gramsmole n 10 g Fe55845 gmol 0179 mol Fe.
N mM where. The same as the mass of one mole of the compound in grams. For example the mass of a mole of carbon is 12g. As a result each mole of iron atoms has a mass of 55845 g. Convert grams Iron to moles or moles Iron to grams Percent composition by element. It dissolves readily in dilute acids.
 Source: pinterest.com
Source: pinterest.com
How many moles are there in 285 grams of iron. Convert grams Iron to moles or moles Iron to grams Percent composition by element. The formula is. Calculate the moles of iron Fe in 10 g of Fe. Gram per mole gmol 5585.
 Source: slideplayer.com
Source: slideplayer.com
It dissolves readily in dilute acids. The answer is 55845. The molar mass of Iron is 55845 gmol. It can be calculated using the elements atomic weight from the periodic table and expressed in grams. 1 10 -6.
 Source: youtube.com
Source: youtube.com
For example iron Fe has a gram atomic mass of 55845 g due to its atomic weight of 55845 u. The same as the mass of one mole of the compound in grams. Various physical properties of Iron are listed below. Hence molar mass of iron is 56g. We know that molar mass of an element is the atomic mass in grams.

Mass of 1 atom 1 atom mass of a mole of atoms 6022 x 10 23 atoms. Terms in this set 9. 5 mole is present in 28g of iron. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements. The molar mass of Iron is 55845 gmol.
 Source: youtube.com
Source: youtube.com
The mole is Avogadros number 6022 X 1023 of atoms molecules ions or other species. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements. M molar mass gmol 55845 gmol Fe atomic weight from periodic table in gramsmole n 10 g Fe55845 gmol 0179 mol Fe. 1 000 000 000 000. The same as the mass of one mole of the compound in grams.
 Source: slideplayer.com
Source: slideplayer.com
115 rows The atomic mass is useful in chemistry when it is paired with the mole concept. P 12 atmospheres. How many Fe atoms are in 250 grams of Fe. Iron molecular weight. You are looking for n mols On the right you know what R and T are.
 Source: slideplayer.com
Source: slideplayer.com
The units of molar mass follow from its definition. A protein whose molecule has an average mass of 64 kDa would have a molar mass of 64 kgmol. This shared value between molar mass and atomic mass applies to all elements. The molar mass of Iron is 55845 gmol. The answer is 55845.
 Source: pinterest.com
Source: pinterest.com
Molecular mass H 2 O 2 x atomic mass of H atomic mass of O 21008 amu 1600 amu 1802 amu So one mole of water 6022 x 10 23 molecules has a mass of 1802 g. Convert grams Iron to moles or moles Iron to grams Percent composition by element. Plug in the atomic mass of carbon to solve for the mass of 1 atom. The mole is Avogadros number 6022 X 1023 of atoms molecules ions or other species. We know that molar mass of an element is the atomic mass in grams.
 Source: toppr.com
Source: toppr.com
You know what P and V are on the left. 1 mole of atoms 60221023 atoms. V 37 Liters. At 20C 68F or 29315K at standard atmospheric pressure. Molar mass of Fe 55845 gmol.
 Source: pinterest.com
Source: pinterest.com
Molar mass of Fe 55845 gmol. This does not count as 1 of your 3 sentences. Molecular mass H 2 O 2 x atomic mass of H atomic mass of O 21008 amu 1600 amu 1802 amu So one mole of water 6022 x 10 23 molecules has a mass of 1802 g. 1 10 -6. Hence molar mass of iron is 56g.
 Source: youtube.com
Source: youtube.com
At room temperature this metal is in the form of ferrite or α. The definition of atomic mass the mole and molar mass are all directly or indirectly related to carbon-12. In other words the molar mass is the total mass of all the atoms in grams that make a mole of a particular molecule. Calculate the mass in grams of 202 atoms of iron Fe 1 mol of Fe has a mass of 5585 g. Molecular mass H 2 O 2 x atomic mass of H atomic mass of O 21008 amu 1600 amu 1802 amu So one mole of water 6022 x 10 23 molecules has a mass of 1802 g.
 Source: youtube.com
Source: youtube.com
One mole is defined as 6022 X 1023. Density of iron is equal to 7 873 kgm³. This shared value between molar mass and atomic mass applies to all elements. 1 000 000 000 000. A protein whose molecule has an average mass of 64 kDa would have a molar mass of 64 kgmol.

Iron weighs 7873 gram per cubic centimeter or 7 873 kilogram per cubic meter ie. First of all you need to explain the formula you are going to use. The molar mass of any element can be determined by finding the atomic mass of the element on the periodic table. Mass of 1 atom 1 atom mass of a mole of atoms 6022 x 10 23 atoms. Going back to 32 S we know that because its molar mass is 3197 grams per mole its atomic mass must be 3197 amu.
 Source: slideplayer.com
Source: slideplayer.com
One mole is defined as 6022 X 1023. We know that molar mass of an element is the atomic mass in grams. One mole abbreviated mol is equal to 602210 23 molecular entities Avogadros number and each element has a different molar mass depending on the weight of 602210 23 of its atoms 1 mole. It can be calculated using the elements atomic weight from the periodic table and expressed in grams. Various physical properties of Iron are listed below.
 Source: convertermaniacs.com
Source: convertermaniacs.com
Calculate the mass in grams of 202 atoms of iron Fe 1 mol of Fe has a mass of 5585 g. First of all you need to explain the formula you are going to use. It rusts in damp air but not in the dry air. Mass of 1 atom 1 atom mass of a mole of atoms 6022 x 10 23 atoms. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title atomic weight of iron in grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.