Your Atomic weight of gold in grams per mole images are available in this site. Atomic weight of gold in grams per mole are a topic that is being searched for and liked by netizens now. You can Download the Atomic weight of gold in grams per mole files here. Get all royalty-free photos and vectors.
If you’re searching for atomic weight of gold in grams per mole images information connected with to the atomic weight of gold in grams per mole interest, you have visit the ideal site. Our site frequently provides you with suggestions for viewing the highest quality video and image content, please kindly search and find more informative video articles and images that match your interests.
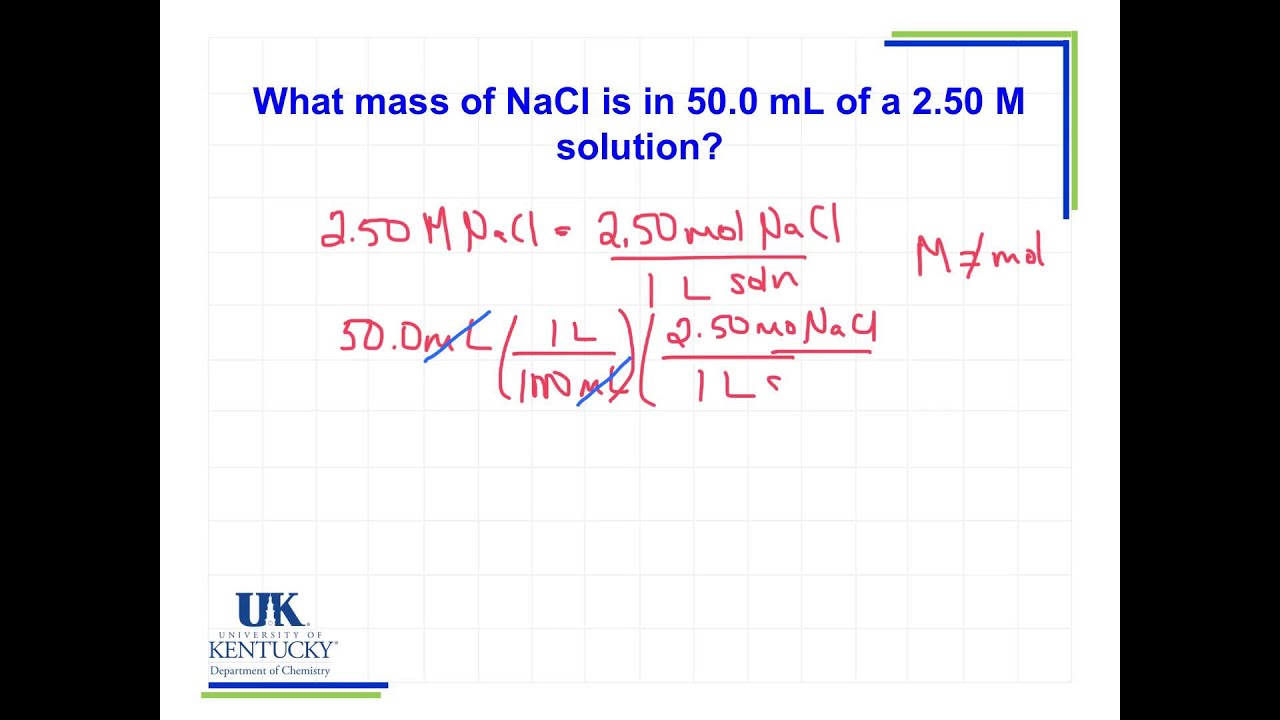
Atomic Weight Of Gold In Grams Per Mole. Compound name is gold. Calculate the number of atoms of Au in 0005077 mol. The same as the mass of one mole of the compound in grams. Divide Avagadros Number by 19696655 to determine the number of atoms in a gram of gold.

The atomic mass of Gold Au is 1969665 grams per mole. The same as the mass of one mole of the compound in grams. This shared value between molar mass and atomic mass applies to all elements. The units for molar mass are grams per mole or gmol. 115 rows The atomic mass is useful in chemistry when it is paired with the mole concept. Au element gold-based compounds Atomic number.
Compound name is gold.
The atomic mass of Gold Au is 1969665 grams per mole. This mass is given by the atomic weight of the chemical unit that makes up that substance in atomic mass units amu. Track your food intake exercise sleep and meditation for free. Convert between Au weight and moles. Mass of 1 C atom 1994 x 10 -23 g. Use this page to learn how to convert between moles Gold and gram.

The atomic mass is useful in chemistry when it is paired with the mole concept. A mole of gold equals about 19696655 grams. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements. Is one mole of cadmium heavier than one mole of gold. Get control of 2021.
 Source: brainly.in
Source: brainly.in
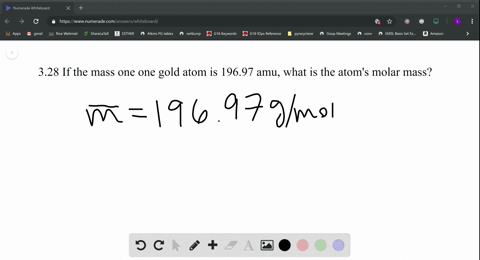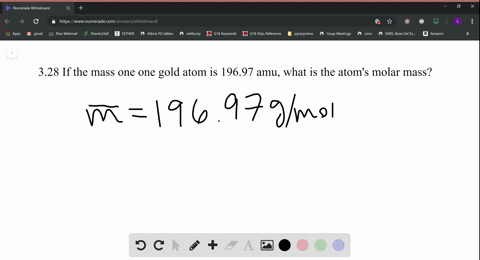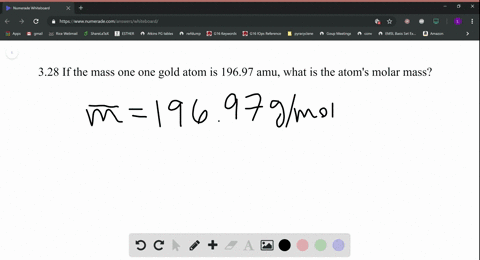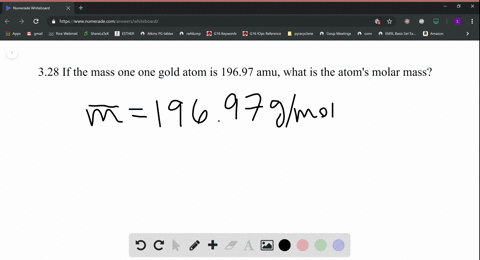
Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements. Atomic mass of Gold is 1969665 u. Atomic Mass of Gold. Gold has an atomic mass of 1079. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element.
 Source: slideplayer.com
Source: slideplayer.com
Convert between Au weight and moles. Mass of 1 C atom 1994 x 10 -23 g. The same as the mass of one mole of the compound in grams. Going back to 32 S we know that because its molar mass is 3197 grams per mole its atomic mass must be 3197 amu. Molar mass is the mass of a given.
 Source: slideplayer.com
Source: slideplayer.com
Use this page to learn how to convert between moles Gold and gram. Note that rounding errors may occur so always check the results. The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units amu or in grams per mole gmol. Therefore 10 grams of carbon is equal to 502 x 1023 atoms. Au element gold-based compounds Atomic number.
 Source: slideplayer.com
Source: slideplayer.com
Molar mass of Au 19696655 gmol. The atomic mass of Gold Au is 1969665 grams per mole. Divide Avagadros Number by 19696655 to determine the number of atoms in a gram of gold. Gold III cation 196964923 gmol grams per mole tetrabromoaurate III anion 51658 gmol grams per mole tetrachloroaurate III anion 3388 gmol grams per mole tetraiodoaurate III anion 704585 gmol grams per mole Image. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.

This shared value between molar mass and atomic mass applies to all elements. The atomic mass of Gold Au is 1969665 grams per mole. Moles n mass mmolar mass M mAu 1 g Au. Mass of 1 atom 1 atom mass of a mole of atoms 6022 x 10 23 atoms. Molar mass of Au 19696655 gmol.
 Source: topperlearning.com
Source: topperlearning.com
This mass is given by the atomic weight of the chemical unit that makes up that substance in atomic mass units amu. Atomic mass of Gold is 1969665 u. Molar mass is the mass of a given. Divide Avagadros Number by 19696655 to determine the number of atoms in a gram of gold. Although mass can be expressed as both amu and gmol gmol is the most useful system of units for laboratory chemistry.
 Source: numerade.com
Source: numerade.com
100 mole of any element has a mass numerically equal to its atomic mass in grams and contains 60221023 particles. For normal samples from earth with typical isotope composition the atomic weight can be approximated by the standard atomic weight or the conventional atomic weight. Gold III cation 196964923 gmol grams per mole tetrabromoaurate III anion 51658 gmol grams per mole tetrachloroaurate III anion 3388 gmol grams per mole tetraiodoaurate III anion 704585 gmol grams per mole Image. 1969665694 Ionization energy eV. N 5988 g 18015 gmol 3324 mol.
 Source: numerade.com
Source: numerade.com
As you already know how the grams to moles conversion work find the number of moles. Gold molecular weight. This mass is given by the atomic weight of the chemical unit that makes up that substance in atomic mass units amu. The atomic mass is useful in chemistry when it is paired with the mole concept. The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units amu or in grams per mole gmol.
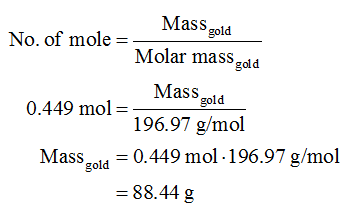 Source: bartleby.com
Source: bartleby.com
100mol of carbon-12 atoms has a mass of 120g and contains 60221023 atoms. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. The same as the mass of one mole of the compound in grams. Mass of 1 atom mass of a mole of atoms 6022 x 10 23. First find the number of moles of Gold in 25 g.
 Source: youtube.com
Source: youtube.com
Atomic Mass of Gold. Compound name is gold. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. 1 mole of atoms 6022 10²³ atoms. The atomic mass is useful in chemistry when it is paired with the mole concept.
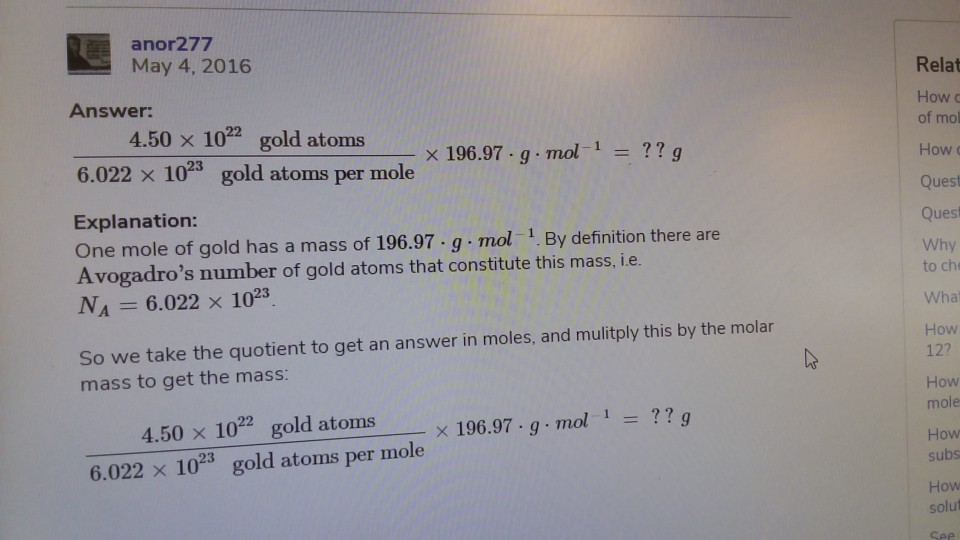 Source: chegg.com
Source: chegg.com
Calculate the number of atoms of Au in 0005077 mol. Is one mole of cadmium heavier than one mole of gold. This mass is given by the atomic weight of the chemical unit that makes up that substance in atomic mass units amu. Gold has an atomic mass of 1079. Its easier to work with grams so convert the mass.
 Source: c.hautarztpraxis-henkel.de
Source: c.hautarztpraxis-henkel.de
100 mole of any element has a mass numerically equal to its atomic mass in grams and contains 60221023 particles. NAu 1 g Au196967 g Aumol Au 0005077 mol Au. For normal samples from earth with typical isotope composition the atomic weight can be approximated by the standard atomic weight or the conventional atomic weight. Get control of 2021. 5988 kg 5988 g.
 Source: nagwa.com
Source: nagwa.com
10 112 602 x 1023 502 x 1023. The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element. Molar mass of gold. Get control of 2021. Mass of 1 C atom 1994 x 10 -23 g.

Is one mole of cadmium heavier than one mole of gold. Is one mole of cadmium heavier than one mole of gold. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. The atomic mass is useful in chemistry when it is paired with the mole concept. N 5988 g 18015 gmol 3324 mol.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title atomic weight of gold in grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.