Your Atomic weight is in grams per mole images are available. Atomic weight is in grams per mole are a topic that is being searched for and liked by netizens today. You can Get the Atomic weight is in grams per mole files here. Get all royalty-free images.
If you’re searching for atomic weight is in grams per mole pictures information related to the atomic weight is in grams per mole topic, you have come to the right site. Our site always gives you hints for refferencing the highest quality video and picture content, please kindly hunt and find more enlightening video content and images that match your interests.
Atomic Weight Is In Grams Per Mole. The corresponding amount-specific mass is MO 16 Daent. Finding molar mass starts with units of grams per mole gmol. Finally we add up the weights of all the atoms to get the total molecular weight of. Where mass is in grams and the molar mass is in grams per mole.
 Gram Atomic Mass 15 P Phosphorus Mole Of An Element Is Equal To Its Atomic Weight Expressed In Grams 1 Mole Of Phosphorus Grams Ppt Download From slideplayer.com
Gram Atomic Mass 15 P Phosphorus Mole Of An Element Is Equal To Its Atomic Weight Expressed In Grams 1 Mole Of Phosphorus Grams Ppt Download From slideplayer.com
Going back to 32 S we know that because its molar mass is 3197 grams per mole its atomic mass must be 3197 amu. Its molar mass is exactly. The units for molar mass are therefore gramsmole. Gram atomic mass is the mass of one mole of an element. Na 2 x 230 460 C 1 x 120 120 O 3 x 160 480 molar mass 1060 gmole For many but not all problems you. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
Finding molar mass starts with units of grams per mole gmol.
Mass number of moles molar mass. So one mole of sodium atoms has a mass of 2299 g. MOLAR MASS OF ATOMS What this means is that the value found on the periodic table is the mass for one mole 602 x 10 23 of atoms for the particular element Also referred to as the atomic weight of an atom We will elaborate more on this later in the course but for now just realize that the units for the mass of atoms will be gmol. Molar mass massmole gmol. An atomic mass is unit less and defined as precisely 112 the mass of an atom of carbon-12 not in motion. Na 2 x 230 460 C 1 x 120 120 O 3 x 160 480 molar mass 1060 gmole For many but not all problems you.
 Source: pinterest.com
Source: pinterest.com
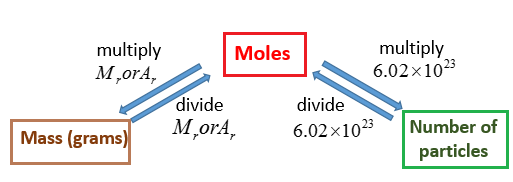
U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. The grams cancel because they are both on the bottom of the equation if you make the amount in grams a fraction over 1 gram which leaves moles and the answer. We can use the above equation to find the mass of a substance when we are given the number of moles of the substance. The units for molar mass are therefore gramsmole. 115 rows The atomic mass is useful in chemistry when it is paired with the mole concept.
 Source: pinterest.com
Source: pinterest.com
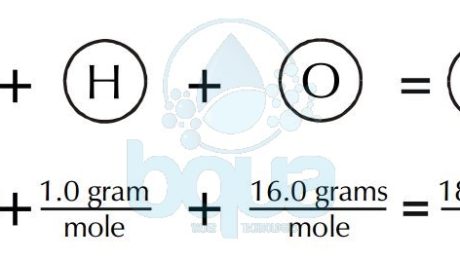
2Molar mass is measured in grams per mole while atomic mass is unitless. And this is exactly equal to 16 gmol or 16 kgkmol. Therefore the mole is a unit for that physical quantity. How many moles are in 1 gram. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams.
 Source: slideplayer.com
Source: slideplayer.com
ArO maODa 16. 2Molar mass is measured in grams per mole while atomic mass is unitless. And this is exactly equal to 16 gmol or 16 kgkmol. There is only 1 oxygen so the total weight of oxygen is 159994. Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight.
 Source: pinterest.com
Source: pinterest.com
This shared value between molar mass and atomic mass applies to all elements. Calculate the mass of a 2 moles and b 025 moles of iron. Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight. Where mass is in grams and the molar mass is in grams per mole. This property simplifies many chemical computations.
 Source: pinterest.com
Source: pinterest.com
Finding molar mass starts with units of grams per mole gmol. An atomic mass is unit less and defined as precisely 112 the mass of an atom of carbon-12 not in motion. Na 2 x 230 460 C 1 x 120 120 O 3 x 160 480 molar mass 1060 gmole For many but not all problems you. 1Molar mass is the mass of one mole per single element while atomic mass is the mass of an atom at rest or is the number of protons and neutrons. And this is exactly equal to 16 gmol or 16 kgkmol.
 Source: bqua.com
Source: bqua.com
So if we first look at carbon carbon we see from this periodic table of elements has a molar mass of 1201 grams per mole. Finally we add up the weights of all the atoms to get the total molecular weight of. Well to figure that out and thats why this periodic table of elements is useful we just have to figure out the molar mass of the constituent elements. So for example sodium Na has an atomic weight of 2299 u so it has a gram atomic mass of 2299 grams. The units for molar mass are therefore gramsmole.
 Source: slideplayer.com
Source: slideplayer.com
The mass of one mole of carbon-12 atoms is exactly 12 grams. Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight. This leads to two important facts. So to convert from grams to moles divide the amount in grams by the atomic weight in grams to find the amount of moles. So one mole of sodium atoms has a mass of 2299 g.
 Source: pinterest.com
Source: pinterest.com
And this is exactly equal to 16 gmol or 16 kgkmol. The units for molar mass are therefore gramsmole. The corresponding amount-specific mass is MO 16 Daent. Mass number of moles molar mass. This leads to two important facts.
 Source: pinterest.com
Source: pinterest.com
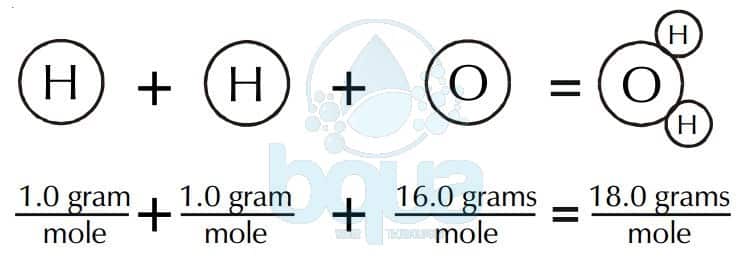
There is only 1 oxygen so the total weight of oxygen is 159994. Finding molar mass starts with units of grams per mole gmol. That is the molar mass of a substance is the mass in grams per mole of 6022 10 23 atoms molecules or formula units of that substanceIn each case the number of grams in 1 mol is the same as the number of atomic mass units that describe the atomic mass the molecular mass or the formula mass respectively. The unity for atomic. Where mass is in grams and the molar mass is in grams per mole.
 Source: pinterest.com
Source: pinterest.com
So one mole of sodium atoms has a mass of 2299 g. While atomic mass is measured in amu molar mass is measured in grams per mole. The mass of one atom of carbon-12 the atomic mass of carbon-12 is exactly 12 atomic mass units. Na 2 x 230 460 C 1 x 120 120 O 3 x 160 480 molar mass 1060 gmole For many but not all problems you. 1Molar mass is the mass of one mole per single element while atomic mass is the mass of an atom at rest or is the number of protons and neutrons.
 Source: bqua.com
Source: bqua.com
There is only 1 oxygen so the total weight of oxygen is 159994. Calculate the mass of a 2 moles and b 025 moles of iron. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. A common request on this site is to convert grams to moles. Therefore the mole is a unit for that physical quantity.
 Source: br.pinterest.com
Source: br.pinterest.com
Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight. Its molar mass is exactly. Moles to Mass Calculation. 115 rows The atomic mass is useful in chemistry when it is paired with the mole concept. Therefore the mole is a unit for that physical quantity.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Where mass is in grams and the molar mass is in grams per mole. Finally we add up the weights of all the atoms to get the total molecular weight of. Since there are 2 hydrogens in the molecule the total weight of hydrogen in water is 2 times 100794 or 201588. 2Molar mass is measured in grams per mole while atomic mass is unitless. The mass of one atom of carbon-12 the atomic mass of carbon-12 is exactly 12 atomic mass units.

Its molar mass is exactly. Na 2 x 230 460 C 1 x 120 120 O 3 x 160 480 molar mass 1060 gmole For many but not all problems you. So for example sodium Na has an atomic weight of 2299 u so it has a gram atomic mass of 2299 grams. This property simplifies many chemical computations. How many moles are in 1 gram.
 Source: pinterest.com
Source: pinterest.com
This leads to two important facts. Gram atomic mass is the mass of one mole of an element. Since the unified atomic mass unit symbol. The grams cancel because they are both on the bottom of the equation if you make the amount in grams a fraction over 1 gram which leaves moles and the answer. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title atomic weight is in grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






