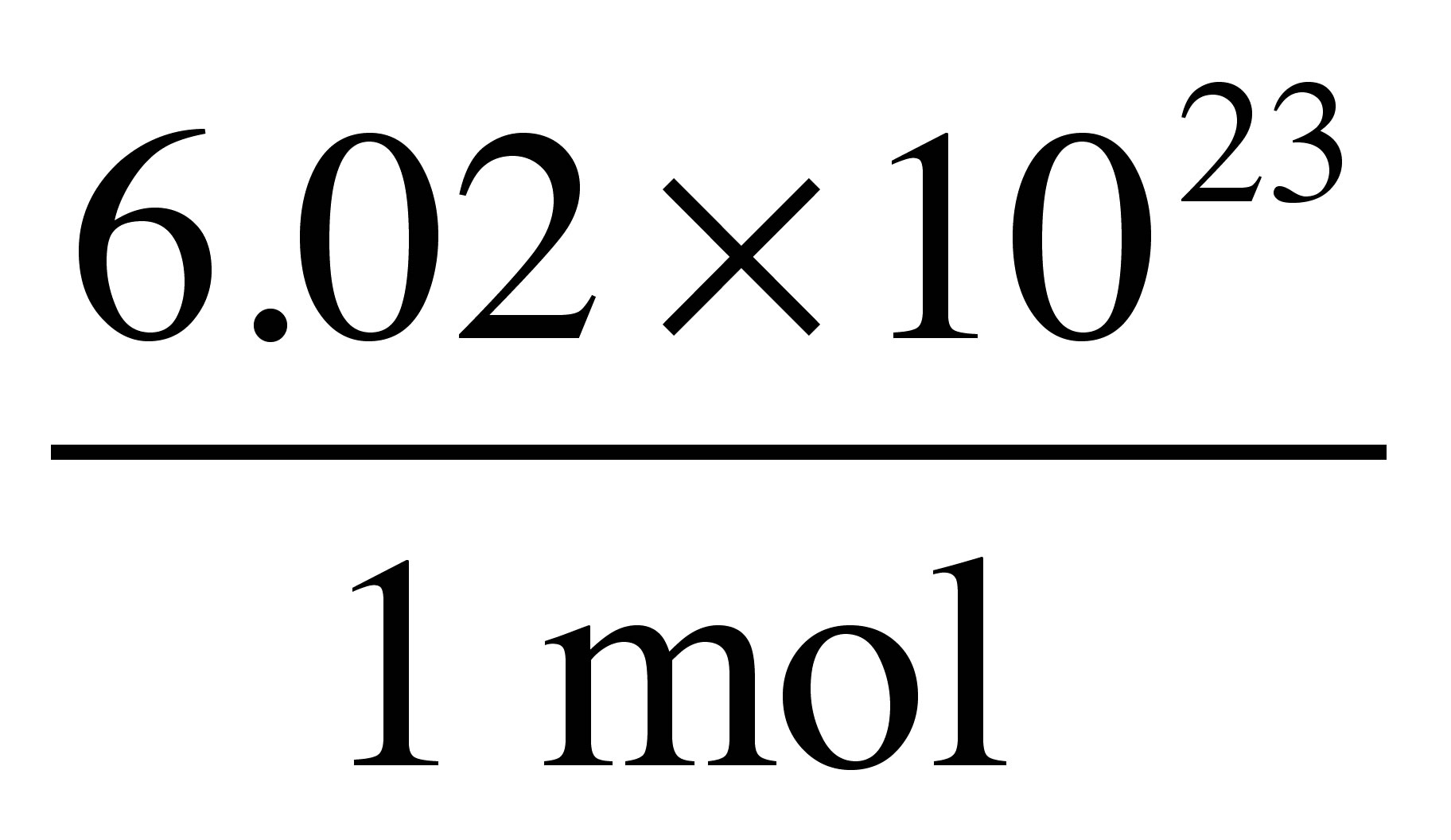Your Atomic weight grams per mole images are ready. Atomic weight grams per mole are a topic that is being searched for and liked by netizens now. You can Download the Atomic weight grams per mole files here. Get all royalty-free photos and vectors.
If you’re looking for atomic weight grams per mole images information linked to the atomic weight grams per mole interest, you have come to the ideal site. Our website always gives you suggestions for downloading the highest quality video and image content, please kindly surf and locate more informative video content and graphics that fit your interests.
Atomic Weight Grams Per Mole. Finding molar mass starts with units of grams per mole gmol. Not to be confused with Molecular Mass. The periodic table shows that the atomic mass rounded to two decimal points of Al is 2698 so 1 mol of Al atoms has a mass of 2698 g. Weve talked about it in other videos you could view this 1201 as a relative atomic mass of a carbon atom of as the average atomic mass of a carbon atom or whats useful and this is where Avogadros Number is valuable if you have Avogadros Number of carbons it is going to have a mass of 1201 grams.
 Atomic Weight Molecular Weight Formula Weight And Molar From slidetodoc.com
Atomic Weight Molecular Weight Formula Weight And Molar From slidetodoc.com
When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. Molecular weight formula helps the molecular mass calculator to find molar mass grams per moles and molecular weight. Convert Inputs to Base Unit Mass percent of the first element. And this is exactly equal to 16 gmol or 16 kgkmol. Weight per weight ww. 120107 grams per mol for carbon 100794 grams per mol for hydrogen and 159994 grams.
The Atomic mass unit u to gram g conversion table and conversion steps are also listed.
Weight per weight ww. While atomic mass is measured in amu molar mass is measured in grams per mole. Convert Inputs to Base Unit Mass percent of the first element. So multiplying 2 hydrogen atoms would yield 2 grams per mole of hydrogen plus 16 grams per mole of oxygen equals 18 grams per mole. The corresponding amount-specific mass is MO 16 Daent. ArO maODa 16.
 Source: br.pinterest.com
Source: br.pinterest.com
From a complete OLI stoichiometry course The molecular weight is the mass of one mole of a substance. Convert Inputs to Base Unit Mass percent of the first element. Measured in Gram Per Mole STEP 1. 112 of the mass of. Going back to 32 S we know that because its molar mass is 3197 grams per mole its atomic mass must be 3197 amu.
 Source: x-engineer.org
Source: x-engineer.org
In this movie we show how to calculate the molecular weight of a substance from the. Gmol to kgmol Conversion. Tables of atomic weights list the numerical values of atomic-scale masses in daltons–eg. Also explore tools to convert Atomic mass unit or gram to other weight and mass units or learn more about weight and mass conversions. 2698 Gram Per Mole – 002698 Kilogram Per Mole Check conversion here Atomic mass of first element.
 Source: pinterest.com
Source: pinterest.com
A hydrogen atom weighs 1 gram per mole. So carbon has a molar mass of 1201 grams per mole and now we can. Weve talked about it in other videos you could view this 1201 as a relative atomic mass of a carbon atom of as the average atomic mass of a carbon atom or whats useful and this is where Avogadros Number is valuable if you have Avogadros Number of carbons it is going to have a mass of 1201 grams. The tabular chart on the right is arranged by Atomic mass weight. ArO maODa 16.
 Source: chemistrygod.com
Source: chemistrygod.com
Measured in Gram Per Mole STEP 1. An oxygen atom weighs 16 grams per mole. From a complete OLI stoichiometry course The molecular weight is the mass of one mole of a substance. The corresponding amount-specific mass is MO 16 Daent. According to the periodic table 1 mol of U has a mass of 23803 g so the mass of 2 mol is twice that.
 Source: slideplayer.com
Source: slideplayer.com
Although the standard SI unit for Molar Mass is kilogram per mol kgmol it is most commonly described with grams per mol gm. This shared value between molar mass and atomic mass applies to all elements. The abbreviation for gmol and kgmol is gram per mole and kilogram per mole respectively. Going back to 32 S we know that because its molar mass is 3197 grams per mole its atomic mass must be 3197 amu. An oxygen atom weighs 16 grams per mole.
 Source: pinterest.com
Source: pinterest.com
So to convert from grams to moles divide the amount in grams by the atomic weight in grams to find the amount of moles. 1 Gram Per Mole is 1000 times Smaller than 1 Oxygen O - Standard Atomic Weight. The abbreviation for gmol and kgmol is gram per mole and kilogram per mole respectively. Usually the units used for this are grams per mole. Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance.
 Source: pinterest.com
Source: pinterest.com
Please note that the elements do not show their natural relation towards each other as in the Periodic system. Measured in Gram Per Mole STEP 1. The molecular weight formula is Molecular Weight number of hydrogen atoms H atomic weight number of oxygen atoms O atomic weight. How many moles are in 1 gram. 115 rows 203 amu.
 Source: pinterest.com
Source: pinterest.com
The Atomic mass unit u to gram g conversion table and conversion steps are also listed. 120107 grams per mol for carbon 100794 grams per mol for hydrogen and 159994 grams. And this is exactly equal to 16 gmol or 16 kgkmol. So to convert from grams to moles divide the amount in grams by the atomic weight in grams to find the amount of moles. 2698 Gram Per Mole – 002698 Kilogram Per Mole Check conversion here Atomic mass of first element.
 Source: pinterest.com
Source: pinterest.com
Although the standard SI unit for Molar Mass is kilogram per mol kgmol it is most commonly described with grams per mol gm. Gram Per Moles to Kilogram Per Moles Converter. Grams of substance in 100 ml of solution mass of solute in gvolume of sol. Weve talked about it in other videos you could view this 1201 as a relative atomic mass of a carbon atom of as the average atomic mass of a carbon atom or whats useful and this is where Avogadros Number is valuable if you have Avogadros Number of carbons it is going to have a mass of 1201 grams. Thus the molecular weight or molar mass of water is 18 grams per mole.
 Source: pinterest.com
Source: pinterest.com
When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. Tables of atomic weights list the numerical values of atomic-scale masses in daltons–eg. Not to be confused with Molecular Mass. Please note that the elements do not show their natural relation towards each other as in the Periodic system. In this movie we show how to calculate the molecular weight of a substance from the.
 Source: slidetodoc.com
Source: slidetodoc.com
1 Gram Per Mole is equal to 0001 Oxygen O - Standard Atomic Weight. Relative Atomic Weight atomic mass unit u. 115 rows 203 amu. Ml of substance in 100 ml of solution volume. How to calculate Molecular Weight.
 Source: pinterest.com
Source: pinterest.com
The atomic mass is useful in chemistry when it is paired with. Units of measurement use the International System of Units better known as SI units which provide a standard for measuring the physical properties of matter. 2698 Gram Per Mole – 002698 Kilogram Per Mole Check conversion here Atomic mass of first element. And this is exactly equal to 16 gmol or 16 kgkmol. Convert Inputs to Base Unit Mass percent of the first element.
 Source: slidetodoc.com
Source: slidetodoc.com
Weve talked about it in other videos you could view this 1201 as a relative atomic mass of a carbon atom of as the average atomic mass of a carbon atom or whats useful and this is where Avogadros Number is valuable if you have Avogadros Number of carbons it is going to have a mass of 1201 grams. So to convert from grams to moles divide the amount in grams by the atomic weight in grams to find the amount of moles. The Atomic mass unit u to gram g conversion table and conversion steps are also listed. There are three elements - carbon hydrogen and oxygen - so find their atomic masses in the periodic table. 1 gmol is 1000 times smaller than a kgmol.
 Source: pinterest.com
Source: pinterest.com
The lightest chemical element is Hydrogen and the heaviest is Hassium. Also explore tools to convert Atomic mass unit or gram to other weight and mass units or learn more about weight and mass conversions. ArO maODa 16. Grams of substance in 100 ml of solution mass of solute in gvolume of sol. Weve talked about it in other videos you could view this 1201 as a relative atomic mass of a carbon atom of as the average atomic mass of a carbon atom or whats useful and this is where Avogadros Number is valuable if you have Avogadros Number of carbons it is going to have a mass of 1201 grams.
 Source: slidetodoc.com
Source: slidetodoc.com
Instant free online tool for Atomic mass unit to gram conversion or vice versa. The mass of one mole of carbon 12 atoms is exactly 12 grams which is its molar mass is exactly 12 grams per mole. The tabular chart on the right is arranged by Atomic mass weight. So carbon has a molar mass of 1201 grams per mole and now we can. Thus the molecular weight or molar mass of water is 18 grams per mole.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title atomic weight grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.