Your Are amu and grams per mole the same images are available. Are amu and grams per mole the same are a topic that is being searched for and liked by netizens today. You can Get the Are amu and grams per mole the same files here. Find and Download all royalty-free images.
If you’re looking for are amu and grams per mole the same pictures information related to the are amu and grams per mole the same topic, you have pay a visit to the ideal site. Our website frequently provides you with hints for refferencing the highest quality video and image content, please kindly surf and find more enlightening video articles and graphics that match your interests.
Are Amu And Grams Per Mole The Same. Okay So some people might say mass is the same as weight only in different units. Relationship Between AMU and Grams The terms amu and grams can be interconverted. This unit is commonly used in physics and chemistry to express the mass of atomic. One amu is equal to one gram per mole.
 1 Mole Of Substance Is Equal To Atomic Molecular Mass In Grams Chemistry Stack Exchange From chemistry.stackexchange.com
1 Mole Of Substance Is Equal To Atomic Molecular Mass In Grams Chemistry Stack Exchange From chemistry.stackexchange.com
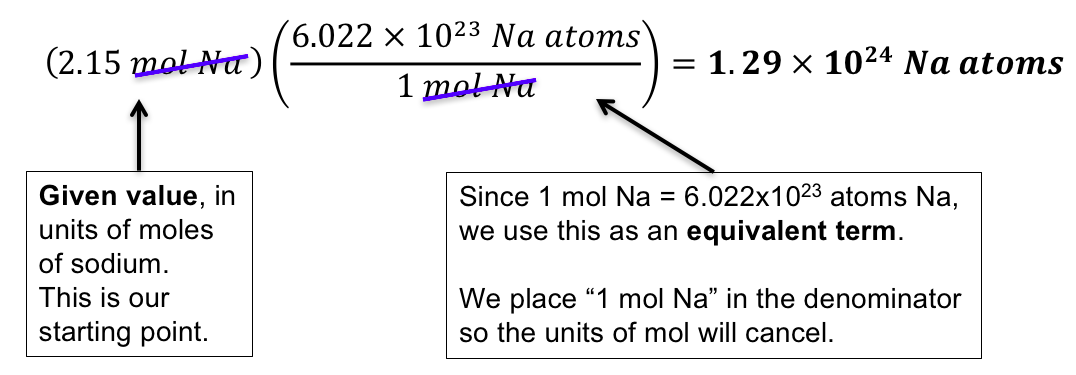
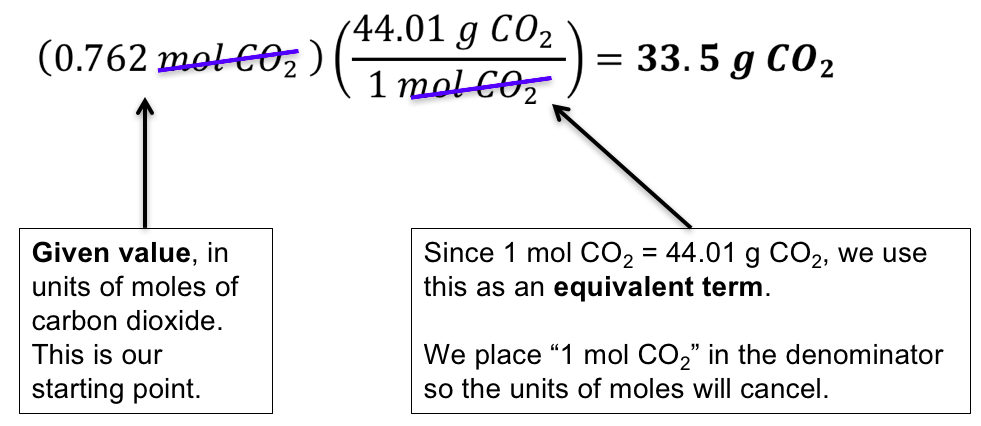
The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units amu or in grams per mole gmol. Atoms and molecule mass is measured in amu. Note that rounding errors may occur so always check the results. One AMU is equivalent to 166 x 10-24 grams. The atomic mass of carbon-12 isotope is 12 amu. One mole of something is equal to 6022140781023 of same things Avogadros number.
Because different molecules and atoms do not have the same mass one mole of one thing does not weigh the same as one mole of something else.
The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units amu or in grams per mole gmol. Molar Mass fracMass of the substances kgAmount of substance mol The SI unit of molar mass is kgmol. Okay So some people might say mass is the same as weight only in different units. Relationship Between AMU and Grams The terms amu and grams can be interconverted. The mass of a single atom of an element amu is numerically equal to the mass g of 1 mol of that element regardless of the element. Atomic Mass Units amu and Grams per Mole gmol can be used interchangeably they are the same size measurement.
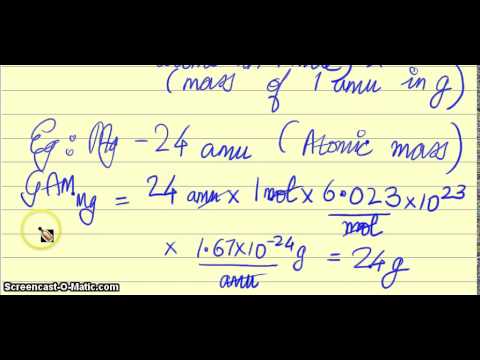 Source: youtube.com
Source: youtube.com
That means one mole of carbon-12 isotope weighs 12 g. Okay So some people might say mass is the same as weight only in different units. This shared value between molar mass and atomic mass applies to all elements. Molecular weight of 7 AmU or mol. Because different molecules and atoms do not have the same mass one mole of one thing does not weigh the same as one mole of something else.
 Source: slideplayer.com
Source: slideplayer.com
The mass of a single atom of an element amu is numerically equal to the mass g of 1 mol of that element regardless of the element. Atomic Mass Units amu are a large scale measurement and Grams per Mole gmol is a small scale measurement c. For a further explanation visit. This unit is commonly used in physics and chemistry to express the mass of atomic. Therefore we just proved that an atomic mass unit is the same thing as grams per mole.
 Source: youtube.com
Source: youtube.com
Atomic Mass Units amu and Grams per Mole gmol can be used interchangeably they are the same size measurement. That is clearly not true because if you go into outer space where there is no force of gravity on you from any object nearby you. For example one mole of grapefruits would be as big as the earth. A very small unit of mass about the mass of a hydrogen atom. In other words the ratio of amuatom is the same as the ratio of gmol.

This means amu can be converted into grams and grams can be converted into amu as well. Molecular weight of 7 AmU or mol. For example one mole of grapefruits would be as big as the earth. That is clearly not true because if you go into outer space where there is no force of gravity on you from any object nearby you. The molecular mass is defined as the mass of one molecule.

Therefore we just proved that an atomic mass unit is the same thing as grams per mole. The molecular mass is defined as the mass of one molecule. One mole of something is equal to 6022140781023 of same things Avogadros number. Because different molecules and atoms do not have the same mass one mole of one thing does not weigh the same as one mole of something else. How small is a Dalton.

1 grams 7 AmU is equal to 00020490589379223 mole. It is measured in amu. The molecular mass is defined as the mass of one molecule. Molar Mass fracMass of the substances kgAmount of substance mol The SI unit of molar mass is kgmol. Its somewhat convenient the relation between grams to amu that is in the sense that the value in amu of a particular atomcompound is pretty much the same with the mass of a mole of that atomcompound.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Grams g are units that are used to measure very large numbers of atoms or molecules. For a further explanation visit. It is a physical property of substances. The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units amu or in grams per mole gmol. One mole of something is equal to 6022140781023 of same things Avogadros number.
 Source: pediaa.com
Source: pediaa.com
The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units amu or in grams per mole gmol. This unit is commonly used in physics and chemistry to express the mass of atomic. This allows us to easily relate masses at the atomic scale to masses at the macroscopic scale. Okay So some people might say mass is the same as weight only in different units. Atoms and molecule mass is measured in amu.
 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
How small is a Dalton. This unit is commonly used in physics and chemistry to express the mass of atomic. Note that rounding errors may occur so always check the results. Molar mass is the mass of a mole of a particular substance means the mass of a particular compound divided by the amount of substance. Use this page to learn how to convert between grams 7 AmU and mole.
 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
Is Amu equal to g mol. The mass of a single atom of an element amu is numerically equal to the mass g of 1 mol of that element regardless of the element. One AMU is equivalent to 166 x 10-24 grams. Atomic mass units amu are units that are used to measure the mass of individual atoms or molecules. One atom of the isotope C-12 was assigned a mass of exactly 12 u.
 Source: quora.com
Source: quora.com
One AMU is equivalent to 166 x 10-24 grams. The molecular mass is defined as the mass of one molecule. This unit is commonly used in physics and chemistry to express the mass of atomic. Use this page to learn how to convert between moles 7 AmU and gram. A very small unit of mass about the mass of a hydrogen atom.
 Source: youtube.com
Source: youtube.com
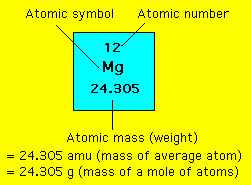
And so mass of carbon-12 12 amuatom. Molar mass is the mass of a mole of a particular substance means the mass of a particular compound divided by the amount of substance. The atomic mass of carbon-12 isotope is 12 amu. Atoms and molecule mass is measured in amu. Atoms and molecule mass.
 Source: cpanhd.sitehost.iu.edu
Source: cpanhd.sitehost.iu.edu
The definitions of amu and moles were intentionally chosen to make that happen Im surprised your teacher didnt explain this actually. Prior to the 2019 redefinition of the SI base units these were numerically identical by definition N A Da 1 gmol and are still treated as such for most purposes. The SI base unit for amount of substance is the mole. For example one mole of grapefruits would be as big as the earth. Okay So some people might say mass is the same as weight only in different units.

1 mole is equal to 1 moles 7 AmU or 48802891 grams. Use this page to learn how to convert between grams 7 AmU and mole. The definitions of amu and moles were intentionally chosen to make that happen Im surprised your teacher didnt explain this actually. Use this page to learn how to convert between moles 7 AmU and gram. In other words the ratio of amuatom is the same as the ratio of gmol.
 Source: slideplayer.com
Source: slideplayer.com
Is amu the same as g mol. Is Amu equal to g mol. The molecular mass is defined as the mass of one molecule. For a further explanation visit. One mole of carbon atoms has a mass of 12011 grams because of the presence of isotopes and because and amu is not exactly the mass of a proton or neutron.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title are amu and grams per mole the same by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






